{Replace with the Title of Your Dissertation}
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

2019 Newsletter
Front page: Allen’s greeting, something new 2019 NEWSLETTER A Message From Our President & Owner, EVENT CALENDAR Cooler mornings and valley fog below the orchard remind us all that it’s about apple time! Nature has blessed us with August 19th a beautiful crop of apples with exceptionally good fruit size. Opening Day Compared to recent years, some varieties may be picked a little later this year so be sure to give us a call or check our website to September 27th - 29th make sure your favorite apple is available. I enjoy every apple Gays Mills Apple Festival variety we grow, but Evercrisp has me as excited as Honeycrisp. October 5th - 6th Harvested in late October and stored in a refrigerator, Evercrisp Sunrise Samples Weekend is a fantastic eating experience in the winter months. Our family has been growing apples since 1934 and we have never tasted October 12th - 13th another winter apple like Evercrisp! Family Fun Weekend I hope you all enjoyed our newly expanded sales area and October 19th - 20th bathrooms added in 2018. This year we have made additional Harvest Celebration exciting improvements with a new gift area, live apple packing & Helicopter Rides TV, and a working model train for young and old to enjoy. Our famous cider donuts will be back- made fresh every day. Please (weather permitting ) enjoy our free apple and cider samples along with many of the October 21st - December 16th other products we sell. Gift Box Shipping Begins Don’t forget our online store. We feature many of the October 26th - 27th items available here and have made it far easier to order gift pack Trick or Treat Weekend apples this year from home. -

Planting and Aftercare of New Trees
Where to start? • Fruit plants that fit into to small spaces Producing Fruit for the Home – Apple … on dwarfing rootstocks • Most traditional and local garden centers do not identify specific rootstock ….”Dwarf”, “Semi Dwarf” Ron Perry • Eventual tree size within Dwarf and Semi Dwarf is large Professor Tree Spacing Nursery ID Hort. Department Rootstocks Eventual Height Between Trees Between Rows MSU M.27 or P.22 Dwarf 6 5 10 M.9 Dwarf 8 8 12 M.26 Dwarf 16 10 16 M.7 Semi Dwarf 18 14 22 MM.106 or 111 Semi Dwarf 20 16 22 Where to start? Where to start? • Fruit plants that fit into to small spaces – Cherry - Sour • Select desired fruit which will grow in your area. Tree Spacing Rootstocks • Determine how much space you have available. Varieties Eventual Height Between Trees Between Rows Northstar Mahaleb 10 8 12 • Select varieties which are easiest to grow. Montmorency Gi.5 or 6 12 10 12 Montmorency Mahaleb 12 10 14 – Disease or insect resistant varieties to reduce pest Montmorency Mazzard 14 12 16 pressures. Balaton Mahaleb 14 12 16 – Cherry - Sweet – Assess soil / site conditions Tree Spacing • Full sun VS shade or partial Nursery ID • Soil internal drainage Rootstocks Eventual Height Between Trees Between Rows • Weed competition (lawns are too competitive) Gi.5 Dwarf 12 12 16 Gi.6 Dwarf 14 14 16 Mahaleb Semi Dwarf 20 14 16 Mazzard Semi Dwarf 24 16 20 Average Annual Minimum Temperatures Where to start? (USDA Plant Hardiness Zone Map) Most MI fruit sites Zone 5 (-20oF to -10oF) to 6 (-10oF to 0oF) • Fruit plants that fit into to small spaces – Peach, Nectarine, Apricot and Plums – Can generally plant at a spacing of 10 ft X 15 ft* • * If trained to open center or vase shape • Closer spacing, needs to be trained in Chistmas Tree form (Vertical Axe). -

Diseases of Tree Fruit Apple: Diagnosis and Management
1 Diseases of Tree Fruit Apple: Diagnosis and Management Sara M. Villani June 22, 2017 Department of Entomology and Plant Pathology, NCSU [email protected] 2 Apple Disease Challenges in the S.E. • Several apple diseases to contend with Apple Disease Challenges in the S.E. 3 • Paucity of disease resistant cultivars – Breeding efforts focus on consumer preference – Usually single-disease resistance ‘Enterprise’ ‘Prima’ ‘Goldrush’ http://www.eatlikenoone.com/prima-apples.htm http://www.eatlikenoone.com/enterpris-apples.htm http://kuffelcreek.wordpress.com/ ‘William’s Pride’ ‘Liberty’ ‘Pristine’ http://www.eatlikenoone.com/pristine-apples.htm http://www.plant.photos.net/index.php?title=File:Apple_williams_pride.jpg http://www.plant.photos.net/index.php?title=File:Apple_libertye.jpg Apple Disease Challenges in the S.E. 4 • Warm, humid climate – Favorable for pathogen infection and disease development – Inadequate chilling hours: longer period of susceptibility to blossom infection Susceptible Host Biology and Conducive availability of Environment pathogen Apple Disease Challenges in the S.E. 5 • Maintaining practices of fungicide resistance management and maximum annual applications – Commercial apple growers in Hendersonville NC: Up to 24 fungicide applications in 2017! Multi-site Single-site Biologicals Protectants Fungicides Mancozeb Group 3: S.I.’s Bacillus spp. Captan Group 11: “Strobys” A. pullulans Copper Group 7: SDHIs Sulfur Group 1: “T-Methyl” ziram U12: Dodine Phosphorous Acid Confusing Fungicide Jargon 6 Fungicides are classified in a number of ways: 1. Chemical Group – e.g. triazoles, benzimidazoles 2. Biochemical Mode of Action (my preference, common in academia) – e.g. Demethylation inhibitor (DMI); Quinone-outside inhibitor (QoI) 3. Physical Mode of Action – e.g. -
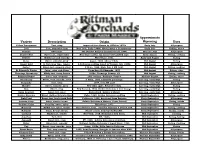
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in Rosbreed
Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in RosBREED Jim Luby, Breeding Team Leader Outline of Presentation RosBREED Demonstration Breeding Programs Standardized Phenotyping Protocols Reference Germplasm Sets SNP Detection Panels Crop Reference Set Breeding Pedigree Set RosBREED Demonstration Breeding Programs Clemson U WSU Texas A&M UC Davis U Minn U Arkansas Rosaceae Cornell U WSU MSU MSU Phenotyping Affiliates USDA-ARS Driscolls Corvallis Univ of Florida UNH Standardized Phenotyping Protocols Traits and Standardized Phenotyping Protocols • Identify critical fruit quality traits and other important traits • Develop standardized phenotyping protocols to enable data pooling across locations/institutions • Protocols available at www.RosBREED.org Apple Standardized Phenotyping Firmness, Crispness – Instrumental, Sensory Sweetness, Acidity – Intstrumental, Sensory Color, Appearance, Juiciness, Aroma – Sensory At harvest Cracking, Russet, Sunburn Storage 10w+7d Storage 20w+7d Maturity Fruit size 5 fruit (reps) per evaluation Postharvest disorders Harvest date, Crop, Dropping RosBREED Apple Phenotyping Locations Wenatchee, WA St Paul, MN Geneva, NY • One location for all evaluations would reduce variation among instruments and evaluators • Local evaluations more sustainable and relevant for future efforts at each institution • Conduct standardized phenotyping of Germplasm Sets at respective sites over multiple (2-3) seasons • Collate data in PBA format, conduct quality control, archive Reference -

Bristol Naturalist News
Contents / Diary of events NOVEMBER 2017 Bristol Naturalist News Discover Your Natural World Bristol Naturalists’ Society BULLETIN NO. 565 NOVEMBER 2017 BULLETIN NO. 565 NOVEMBER 2017 Bristol Naturalists’ Society Discover Your Natural World Registered Charity No: 235494 www.bristolnats.org.uk HON. PRESIDENT: Andrew Radford, Professor CONTENTS of Behavioural Ecology, Bristol University 3 Diary of Events ACTING CHAIRMAN: Stephen Fay HON. PROCEEDINGS RECEIVING EDITOR: 4 Society Walk / Society Talk Dee Holladay, 15 Lower Linden Rd., Clevedon, 5 Lesley’s “Natty News…” BS21 7SU [email protected] HON. SEC.: Lesley Cox 07786 437 528 6 Get Published! Write for Nature in Avon [email protected] HON. MEMBERSHIP SEC: Mrs. Margaret Fay 7 Joint BNS/University programme 81 Cumberland Rd., BS1 6UG. 0117 921 4280 8 Phenology ; Book Club [email protected] Welcome to new members HON. TREASURER: Michael Butterfield 14 Southdown Road, Bristol, BS9 3NL 9 Society Walk Report; (0117) 909 2503 [email protected] Poem for the month BULLETIN DISTRIBUTION 10 BOTANY SECTION Hand deliveries save about £800 a year, so help Botanical notes; Meeting Report; is much appreciated. Offers please to: Plant Records HON. CIRCULATION SEC.: Brian Frost, 60 Purdy Court, New Station Rd, Fishponds, Bristol, BS16 13 GEOLOGY SECTION 3RT. 0117 9651242. [email protected] He will be pleased to supply further details. Also 14 INVERTEBRATE SECTION Notes for this month contact him about problems with (non-)delivery. BULLETIN COPY DEADLINE: 7th of month before 15 LIBRARY Hand-coloured books publication to the editor: David B Davies, The Summer House, 51a Dial Hill Rd., Clevedon, 17 ORNITHOLOGY SECTION BS21 7EW. -

Cedar-Apple Rust
DIVISION OF AGRICULTURE RESEARCH & EXTENSION Agriculture and Natural Resources University of Arkansas System FSA7538 Cedar-Apple Rust Stephen Vann Introduction Assistant Professor One of the most spectacular Extension Urban Plant Pathologist diseases to appear in spring is cedar- apple rust. This disease is caused by the fungus Gymnosporangium juniperi-virginianae and requires both cedar and apple trees to survive each year. It is mainly a problem in the eastern portion of North America and is most important on apple or crab Figure 2. Cedar-apple rust on crabapple apple (Malus sp), but can also affect foliage. quince and hawthorn. yellow-orange color (Figures 1 and 2). Symptoms On the upper leaf surface of these spots, the fungus produces specialized The chief damage by this disease fruiting bodies called spermagonia. On occurs on apple trees, causing early the lower leaf surface (and sometimes leaf drop and poor quality fruit. This on fruit), raised hair-like fruiting bod can be a significant problem to com ies called aecia (Figure 3) appear as mercial apple growers but also harms microscopic cup-shaped structures. the appearance of ornamental crab Wet, rainy weather conditions favor apples in the home landscape. On severe infection of the apple. The apple, symptoms first appear as fungus forms large galls on cedar trees small green-yellow leaf or fruit spots in the spring (see next section), but that gradually enlarge to become a these structures do not greatly harm Arkansas Is Our Campus Visit our web site at: Figure 1. Cedar-apple rust (leaf spot) on Figure 3. Aecia of cedar-apple rust on https://www.uaex.uada.edu apple (courtesy J. -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion -

Open KON Dissertation FINAL.Pdf
The Pennsylvania State University The Graduate School College of Agricultural Sciences EVALUATION OF CHEMICAL AND THERMAL BLOSSOM THINNING STRATEGIES FOR APPLE A Dissertation in Horticulture by Thomas M. Kon 2016 Thomas M. Kon Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2016 The dissertation of Thomas M. Kon was reviewed and approved* by the following: James R. Schupp Professor of Pomology Dissertation Advisor Chair of Committee Robert M. Crassweller Professor of Horticulture Paul Heinemann Professor and Head of Agricultural and Biological Engineering Richard Marini Professor of Horticulture Erin L. Connolly Professor of Plant Science Head of the Department of Plant Science *Signatures are on file in the Graduate School iii ABSTRACT Since 1989, approximately 150 compounds and multiple mechanical devices were evaluated as apple blossom thinners. Despite these efforts, blossom thinner adoption has been limited to a few apple producing regions or states. The purpose of this work was to: 1) compare the efficacy of promising chemical blossom thinners using a predictive model as a timing aid, and 2) evaluate the potential of short-duration thermal treatments as a blossom thinning strategy. Using a predictive model as a timing aid, the efficacy of several promising chemical blossom thinners was evaluated at Penn State’s Fruit Research and Extension Center in Biglerville, PA. Blossom thinner effects on pollen tube growth, fruit set, and yield responses were evaluated. Calcium polysulfide and ammonium thiosulfate inhibited pollen tube growth in vivo and reduced initial fruit set. Endothal was a potent thinner, but was ineffective in reducing pollen tube growth and caused excessive leaf injury. -

Systematically Integrating DNA Information Into Breeding: the MAB
Systematically integrating DNA information into breeding: The MAB Pipeline, case studies in apple and cherry Amy Iezzoni January 31, 2013 Cornell MSU Susan Brown Amy Iezzoni (PD) Kenong Xu Jim Hancock Dechun Wang Clemson Cholani Weebadde Ksenija Gasic Gregory Reighard Univ. of Arkansas John Clark WSU Texas A&M USDA-ARS Dave Byrne Cameron Peace Nahla Bassil Dorrie Main Univ. of Minnesota Gennaro Fazio Univ. of CA-Davis Kate Evans Chad Finn Karina Gallardo Jim Luby Tom Gradziel Vicki McCracken Chengyan Yue Plant Research Intl, Carlos Crisosto Nnadozie Oraguzie Netherlands Oregon State Univ. Eric van de Weg Univ. of New Hamp. Alexandra Stone Marco Bink Tom Davis Outline of Presentation The MAB Pipeline Apple skin color Cherry flesh color The MAB Pipeline “Jewels in the Genome” - discovering, polishing, applying QTL discovery MAB Pipelining Breeding (looks promising...) (polishing...) (assembling into masterpieces) Socio-Economics Surveys (example for apple) Washington Michigan Market Breeders Producers Producers Intermediaries Fruit flavor 43 41 23 Fruit crispness 15 23 10 Exterior color 26 Fruit firmness 6 7 5 Shelf life at retail 7 7 3 Sweetness/soluble solids 6 7 3 Sugar/acid balance 9 7 External appearance 13 No storage disorders 7 4 Disease resistance 2 5 Storage life 5 Other fruit quality…2 3 Size 3 Juiciness 2 Tartness Shape Phytonutrient Aroma % of respondents020406080100 Reference Germplasm McIntosh Melba LivelRasp Jolana Williams F_Spartan Spartan PRI14-126 Starr OR38T610 F_Williams NJ53 PRI14-226 Minnesota Delicious KidsOrRed -

Friday, September 4, 2020 WEEK AHEAD WEEK BEYOND
SAFETY/SECURITY COMMUNICATIONS. The Communications Center received 648 calls for police service, 31 fire calls and 62 EMS calls for a total number of calls received of 741. There were 11 male inmates and 8 female inmates housed in our jail. FIRE. Fire companies made 31 fire calls and 55 EMS calls. Of the EMS patients, 43 were transports and 12 were non-transports. They performed 5 building inspections. The Fire Marshal’s Office conducted 16 building inspections, 2 fire investigations and performed 3 plans reviews. The City Emergency Management Team continues to meet to respond to the impact from Coronavirus (COVID-19). On Tuesday, TN-TF1 was activated as a Type 4 Water Rescue Team to deploy to Louisiana for Hurricane Laura, which made landfall on Thursday as a Major Category 4 Hurricane. Administrative Chief Keith Saunders, Lieutenant Steven Mosby, and IT Director/Reserve Fire Lieutenant Tony Fischer deployed with the team. They remain working in the Lake Charles and Calcasieu Parrish in Louisiana, which was the hardest hit area. They are conducting wide area searches of the damaged buildings and neighborhoods, including providing support for survivors. Congratulations to Wayne Hampton and Nick Signaigo! They were promoted to Lieutenant following a very competitive promotional process. They fill open Friday, September 4, 2020 positions due to retirements of Tony Hulbert and Mike Pohl. WEEK AHEAD INVESTIGATIONS. The Investigations Division had 29 reports assigned, cleared 22 reports, Tuesday, September 8, 5:30 p.m. (Digital) issued 0 warrants and made 0 arrests. Beautification Commission, PR- Sanders Tuesday, September 8, 6:00p.m.