Recognition and Distribution of Two North Atlantic Gadiculus Species, G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Interspecific Variation in the Intestinal Microbiomes Of
bioRxiv preprint doi: https://doi.org/10.1101/864439; this version posted December 5, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Metagenomic shotgun analyses reveal complex patterns of 2 intra- and interspecific variation in the intestinal 3 microbiomes of codfishes 4 5 6 Even Sannes Riiser1*, Thomas H.A. Haverkamp1,2, Srinidhi Varadharajan1, 7 Ørnulf Borgan3, Kjetill S. Jakobsen1, Sissel Jentoft1 and Bastiaan Star1* 8 9 1Centre for Ecological and Evolutionary Synthesis, Department of Biosciences, 10 University of Oslo, PO Box 1066, Blindern, N-0316 Oslo, Norway. 11 12 2Present Address: Department of Epidemiology, Norwegian Veterinary Institute, 13 Oslo, Norway 14 15 3Department of Mathematics, University of Oslo, PO Box 1053, Blindern, N- 16 0316 Oslo, Norway. 17 18 19 * Correspondence 20 Even Sannes Riiser*: [email protected] 21 Bastiaan Star*: [email protected] 22 23 24 Abstract 25 The relative importance of host-specific selection or environmental factors in 26 determining the composition of the intestinal microbiome in wild vertebrates 27 remains poorly understood. Here, we use metagenomic shotgun sequencing of 28 individual specimens to compare the intra- and interspecific variation of 29 intestinal microbiome communities in two ecotypes (NEAC and NCC) of 30 Atlantic cod (Gadus morhua) –that have distinct behavior and habitats– and 31 three Gadidae species that occupy a range of ecological niches. Interestingly, we 32 find significantly diverged microbiomes amongst the two Atlantic cod ecotypes. -

Molecular Systematics of Gadid Fishes: Implications for the Biogeographic Origins of Pacific Species
Color profile: Disabled Composite Default screen 19 Molecular systematics of gadid fishes: implications for the biogeographic origins of Pacific species Steven M. Carr, David S. Kivlichan, Pierre Pepin, and Dorothy C. Crutcher Abstract: Phylogenetic relationships among 14 species of gadid fishes were investigated with portions of two mitochondrial DNA (mtDNA) genes, a 401 base pair (bp) segment of the cytochrome b gene, and a 495 bp segment of the cytochrome oxidase I gene. The molecular data indicate that the three species of gadids endemic to the Pacific Basin represent simultaneous invasions by separate phylogenetic lineages. The Alaskan or walleye pollock (Theragra chalcogramma) is about as closely related to the Atlantic cod (Gadus morhua) as is the Pacific cod (Gadus macrocephalus), which suggests that T. chalcogramma and G. macrocephalus represent separate invasions of the Pacific Basin. The Pacific tomcod (Microgadus proximus) is more closely related to the Barents Sea navaga (Eleginus navaga) than to the congeneric Atlantic tomcod (Microgadus tomcod), which suggests that the Pacific species is derived from the Eleginus lineage and that Eleginus should be synonymized with Microgadus. Molecular divergences between each of the three endemic Pacific species and their respective closest relatives are similar and consistent with contemporaneous speciation events following the reopening of the Bering Strait ca. 3.0–3.5 million years BP. In contrast, the Greenland cod (Gadus ogac) and the Pacific cod have essentially identical mtDNA sequences; differences between them are less than those found within G. morhua. The Greenland cod appears to represent a contemporary northward and eastward range extension of the Pacific cod, and should be synonymized with it as G. -

Histological Aspects of the Early Development of the Digestive System of Burbot Lota Lota L
ISSN 0015-5497, e-ISSN 1734-9168 Folia Biologica (Kraków), vol. 64 (2016), No 1 Ó Institute of Systematics and Evolution of Animals, PAS, Kraków, 2015 doi:10.3409/fb64_1.11 Histological Aspects of the Early Development of the Digestive System of Burbot Lota lota L. (Lotidae, Gadiformes)* Gra¿yna FURGA£A-SELEZNIOW, Ma³gorzata JANKUN, Roman KUJAWA, Joanna NOWOSAD, Maria BI£AS, Dariusz KUCHARCZYK, and Andrzej SKRZYPCZAK Accepted December 04, 2015 Published January 29, 2016 FURGA£A-SELEZNIOW G., JANKUN M., KUJAWA R., NOWOSAD J., BI£AS M., KUCHARCZYK D., SKRZYPCZAK A. 2016. Histological aspects of the early development of the digestive system of burbot Lota lota L. (Lotidae, Gadiformes). Folia Biologica (Kraków) 64: 11-21. The ontogeny of the digestive tract was studied histologically in burbot, Lota lota L., from hatching to 42 days post-hatch (dph). At hatching, the digestive tract consisted of a straight tube with discernible digestive accessory glands (the liver and the pancreas) dorsally attached to the yolk sac. Most of the yolk sac reserves were consumed during the first 12 days and were completely depleted by 17 dph. The first PAS-positive goblet cells appeared at 6 dph, dispersed within the epithelium of the oesophagus and increasing substantially in number and distribution as development progressed. At 12 dph, the first vacuoles (neutral lipids) appeared in the intestine, indicating the functional absorption of nutrients from food. Differentiation of gastric glands was first noticed at 17 dph and was extensive by 27 dph. L. lota larvae have a morphologically complete digestive tract by 32 dph. -

The First Evidence of Intrinsic Epidermal Bioluminescence Within Ray-Finned Fishes in the Linebelly Swallower Pseudoscopelus Sagamianus (Chiasmodontidae)
Received: 10 July 2019 Accepted: 22 October 2019 DOI: 10.1111/jfb.14179 BRIEF COMMUNICATION FISH The first evidence of intrinsic epidermal bioluminescence within ray-finned fishes in the linebelly swallower Pseudoscopelus sagamianus (Chiasmodontidae) Michael J. Ghedotti1,2 | W. Leo Smith3 | Matthew P. Davis4 1Department of Biology, Regis University, Denver, Colorado, USA Abstract 2Bell Museum of Natural History, University of External and histological examination of the photophores of the linebelly swallower Minnesota, St. Paul, Minnesota, USA Pseudoscopelus sagamianus reveal three epidermal layers of cells that form the light- 3Department of Ecology and Evolutionary Biology and Biodiversity Institute, University producing and light-transmitting components of the photophores. Photophores of Kansas, Lawrence, Kansas, USA among the examined photophore tracts are not significantly different in structure but 4 Department of Biological Sciences, St. Cloud the presence of mucous cells in the superficial layers of the photophore suggest con- State University, St. Cloud, Minnesota, USA tinued function of the epidermal photophore in contributing to the mucous coat. This Correspondence is the first evidence of intrinsic bioluminescence in primarily epidermal photophores Michael J. Ghedotti, Department of Biology, Regis University, 3333 Regis Boulevard, reported in ray-finned fishes. Denver, CO, 80221-1099, USA. Email: [email protected] KEYWORDS Funding information bioluminescence, deep-sea, histology, integument, photophores, Pseudoscopelus sagamianus, The work primarily was supported by funding Scombriformes from a Regis URSC Grant to M.J.G., a University of Kansas GRF allocation (#2105077) to W.L.S. and National Science Foundation grants (DEB 1258141 and DEB 1543654) to M.P.D. and W.L.S. provided monetary support. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Marine Fishes of the Azores: an Annotated Checklist and Bibliography
MARINE FISHES OF THE AZORES: AN ANNOTATED CHECKLIST AND BIBLIOGRAPHY. RICARDO SERRÃO SANTOS, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS SANTOS, RICARDO SERRÃO, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS 1997. Marine fishes of the Azores: An annotated checklist and bibliography. Arquipélago. Life and Marine Sciences Supplement 1: xxiii + 242pp. Ponta Delgada. ISSN 0873-4704. ISBN 972-9340-92-7. A list of the marine fishes of the Azores is presented. The list is based on a review of the literature combined with an examination of selected specimens available from collections of Azorean fishes deposited in museums, including the collection of fish at the Department of Oceanography and Fisheries of the University of the Azores (Horta). Personal information collected over several years is also incorporated. The geographic area considered is the Economic Exclusive Zone of the Azores. The list is organised in Classes, Orders and Families according to Nelson (1994). The scientific names are, for the most part, those used in Fishes of the North-eastern Atlantic and the Mediterranean (FNAM) (Whitehead et al. 1989), and they are organised in alphabetical order within the families. Clofnam numbers (see Hureau & Monod 1979) are included for reference. Information is given if the species is not cited for the Azores in FNAM. Whenever available, vernacular names are presented, both in Portuguese (Azorean names) and in English. Synonyms, misspellings and misidentifications found in the literature in reference to the occurrence of species in the Azores are also quoted. The 460 species listed, belong to 142 families; 12 species are cited for the first time for the Azores. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -

Marine Fishes from Galicia (NW Spain): an Updated Checklist
1 2 Marine fishes from Galicia (NW Spain): an updated checklist 3 4 5 RAFAEL BAÑON1, DAVID VILLEGAS-RÍOS2, ALBERTO SERRANO3, 6 GONZALO MUCIENTES2,4 & JUAN CARLOS ARRONTE3 7 8 9 10 1 Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca 11 e Asuntos Marítimos, Rúa do Valiño 63-65, 15703 Santiago de Compostela, Spain. E- 12 mail: [email protected] 13 2 CSIC. Instituto de Investigaciones Marinas. Eduardo Cabello 6, 36208 Vigo 14 (Pontevedra), Spain. E-mail: [email protected] (D. V-R); [email protected] 15 (G.M.). 16 3 Instituto Español de Oceanografía, C.O. de Santander, Santander, Spain. E-mail: 17 [email protected] (A.S); [email protected] (J.-C. A). 18 4Centro Tecnológico del Mar, CETMAR. Eduardo Cabello s.n., 36208. Vigo 19 (Pontevedra), Spain. 20 21 Abstract 22 23 An annotated checklist of the marine fishes from Galician waters is presented. The list 24 is based on historical literature records and new revisions. The ichthyofauna list is 25 composed by 397 species very diversified in 2 superclass, 3 class, 35 orders, 139 1 1 families and 288 genus. The order Perciformes is the most diverse one with 37 families, 2 91 genus and 135 species. Gobiidae (19 species) and Sparidae (19 species) are the 3 richest families. Biogeographically, the Lusitanian group includes 203 species (51.1%), 4 followed by 149 species of the Atlantic (37.5%), then 28 of the Boreal (7.1%), and 17 5 of the African (4.3%) groups. We have recognized 41 new records, and 3 other records 6 have been identified as doubtful. -
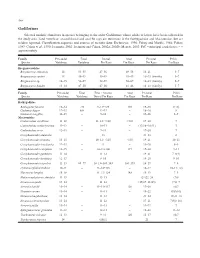
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Copyrighted Material
06_250317 part1-3.qxd 12/13/05 7:32 PM Page 15 Phylum Chordata Chordates are placed in the superphylum Deuterostomia. The possible rela- tionships of the chordates and deuterostomes to other metazoans are dis- cussed in Halanych (2004). He restricts the taxon of deuterostomes to the chordates and their proposed immediate sister group, a taxon comprising the hemichordates, echinoderms, and the wormlike Xenoturbella. The phylum Chordata has been used by most recent workers to encompass members of the subphyla Urochordata (tunicates or sea-squirts), Cephalochordata (lancelets), and Craniata (fishes, amphibians, reptiles, birds, and mammals). The Cephalochordata and Craniata form a mono- phyletic group (e.g., Cameron et al., 2000; Halanych, 2004). Much disagree- ment exists concerning the interrelationships and classification of the Chordata, and the inclusion of the urochordates as sister to the cephalochor- dates and craniates is not as broadly held as the sister-group relationship of cephalochordates and craniates (Halanych, 2004). Many excitingCOPYRIGHTED fossil finds in recent years MATERIAL reveal what the first fishes may have looked like, and these finds push the fossil record of fishes back into the early Cambrian, far further back than previously known. There is still much difference of opinion on the phylogenetic position of these new Cambrian species, and many new discoveries and changes in early fish systematics may be expected over the next decade. As noted by Halanych (2004), D.-G. (D.) Shu and collaborators have discovered fossil ascidians (e.g., Cheungkongella), cephalochordate-like yunnanozoans (Haikouella and Yunnanozoon), and jaw- less craniates (Myllokunmingia, and its junior synonym Haikouichthys) over the 15 06_250317 part1-3.qxd 12/13/05 7:32 PM Page 16 16 Fishes of the World last few years that push the origins of these three major taxa at least into the Lower Cambrian (approximately 530–540 million years ago). -

Length–Weight Relationships of 216 North Sea Benthic Invertebrates
Journal o f the Marine Biological Association o f the United 2010, Kingdom, 90(1), 95-104. © Marine Biological Association of the United Kingdom, 2010 doi:io.ioi7/Soo25 315409991408 Length-weight relationships of 216 North Sea benthic invertebrates and fish L.A. ROBINSON1, S.P.R. GREENSTREET2, H. REISS3, R. CALLAWAY4, J. CRAEYMEERSCH5, I. DE BOOIS5, S. DEGRAER6, S. EHRICH7, H.M. FRASER2, A. GOFFIN6, I. KRÖNCKE3, L. LINDAL JORGENSON8, M.R. ROBERTSON2 AND J. LANCASTER4 School of Biological Sciences, Ecosystem Dynamics Group, University of Liverpool, Liverpool, L69 7ZB, UK, fish eries Research Services, Marine Laboratory, PO Box 101, Aberdeen, AB11 9DB, UK, 3Senckenberg Institute, Department of Marine Science, Südstrand 40,26382 Wilhelmshaven, Germany, 4University of Wales, Swansea, Singleton Park, Swansea, SA2 8PP, UK, Netherlands Institute for Fisheries Research (IMARES), PO Box 77, 4400 AB Yerseke, The Netherlands, sGhent University, Department of Biology, Marine Biology Section, K.L. Ledeganckstraat 35, B 9000, Gent, Belgium, 7Federal Research Institute for Rural Areas, Forestry and Fisheries, Institute of Sea Fisheries, Palmaille 9, 22767 Hamburg, Germany, institute of Marine Research, Box 1870, 5817 Bergen, Norway Size-based analyses of marine animals are increasingly used to improve understanding of community structure and function. However, the resources required to record individual body weights for benthic animals, where the number of individuals can reach several thousand in a square metre, are often prohibitive. Here we present morphometric (length-weight) relationships for 216 benthic species from the North Sea to permit weight estimation from length measurements. These relationships were calculated using data collected over two years from 283 stations. -

Mediterranean Sea
OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi MEDITERRANEAN The IUCN Red List of Threatened Species™ – Regional Assessment OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi The IUCN Red List of Threatened Species™ – Regional Assessment Compilers: Dania Abdul Malak Mediterranean Species Programme, IUCN Centre for Mediterranean Cooperation, calle Marie Curie 22, 29590 Campanillas (Parque Tecnológico de Andalucía), Málaga, Spain Suzanne R. Livingstone Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, c/o Conservation International, Arlington, VA 22202, USA David Pollard Applied Marine Conservation Ecology, 7/86 Darling Street, Balmain East, New South Wales 2041, Australia; Research Associate, Department of Ichthyology, Australian Museum, Sydney, Australia Beth A. Polidoro Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Annabelle Cuttelod Red List Unit, IUCN Species Programme, 219c Huntingdon Road, Cambridge CB3 0DL,UK Michel Bariche Biology Departement, American University of Beirut, Beirut, Lebanon Murat Bilecenoglu Department of Biology, Faculty of Arts and Sciences, Adnan Menderes University, 09010 Aydin, Turkey Kent E. Carpenter Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Bruce B.