The Potential Value of Camera-Trap Studies for Identifying, Ageing, Sexing and Studying the Phenology of Bornean Lophura Pheasants JOHANNES H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Bird Checklists of the World Country Or Region: Myanmar
Avibase Page 1of 30 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Myanmar 2 Number of species: 1088 3 Number of endemics: 5 4 Number of breeding endemics: 0 5 Number of introduced species: 1 6 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Myanmar. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=mm [23/09/2021]. Make your observations count! Submit your data to ebird. -
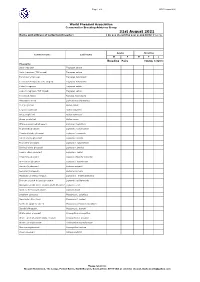
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

1 SYSTEMATIC LIST Red Junglefowl 1 Tabin Crestless Fireback
1 SYSTEMATIC LIST Red Junglefowl 1 Tabin Crestless Fireback 1Cameron Highlands Crested Fireback 1 Tabin Storm's Stork Seen regularly at Uncle Tan’s and 1 at Tabin Glossy Ibis 2 in the Botanic Gardens, KL Black-crowned Night Heron 1 on the Kinabatangan River Striated Heron Widespread Chinese Pond-Heron 1 in the Botanic Gardens, KL Grey Heron 1 at Kuala Selangor Purple Heron Widespread Great White Egret Widespread Little Egret Common on the Kinabatangan River Oriental Darter Frequent on the Kinabatangan River Jerdon's Baza 1 on Common on the Kinabatangan River Oriental Honey-buzzard 1 Cameron Highlands Black-shouldered Kite 1 near Kuala Selangor Brahminy Kite Widespread White-bellied Sea Eagle Common on the Kinabatangan River Lesser Fish-Eagle Common on the Kinabatangan River Grey-headed Fish-Eagle Common on the Kinabatangan River Crested Serpent-Eagle 1 Cameron Highlands, several Uncle Tan’s and Tabin Crested Goshawk 1 on the Kinabatangan River (Indian) Black Eagle 1 at Tabin Changeable Hawk-Eagle Seen twice at Tabin Wallace's Hawk-Eagle Seen twice on Kinabatangan River White-breasted Waterhen Widespread Common Sandpiper Several seen on Kinabatangan River, 1 at Tabin Black-naped Tern Several over the sea at KL airport (Feral) Rock Pigeon Common around KL Spotted-necked (Turtle) Dove Widespread Emerald Dove Seen twice at Tabin Peaceful Dove Common round KL Little Green-Pigeon Seen 4 times in trees on Kinabatangan River Pink-necked Green-Pigeon 1 seen in KL, 1 in trees on Kinabatangan River Large Green Pigeon 1 at Tabin Green Imperial Pigeon -

Remembering Dr. Arthur Crane Risser by Josef Lindholm III, It Stayed That Way Through the Rest of the 1960’S
Remembering Dr. Arthur Crane Risser by Josef Lindholm III, it stayed that way through the rest of the 1960’s. On Dec. 31, Senior Aviculturist, The Dallas World Aquarium 1969, it reached an all-time high of 1,126 species and subspecies of birds (and 3,465 specimens). Then it dropped. At the end of Art Risser’s death following a stroke on the day after Christ- 1970 there were 1,097 taxa. On Jan. 1, 1972, there were 917. A mas 2008, was entirely unexpected. But many of his saddened year later there were 856. And on Jan1., 1974, the number stood friends were also startled to learn he was 70. I think most of us at 772. I found this deeply disturbing. thought he was far younger. When I first met him, shortly after At the same time, my own small avicultural world had also his arrival at the San Diego Zoo, as Assistant Curator of Birds, become much smaller. In 1972, I was, with much effort, able to in 1974, I thought he was in his late twenties. He was, in fact, 35 convince my parents to buy me Red-eared Waxbills at Wool- when he thus entered the zoo profession, having previously been worth’s and Strawberry Finches and Cut-throats at the White involved in mammalogy. Front, all for $3.95 a pair. In 1974, I found the prices for all of He earned his Master’s in Wildlife Management from the these were now $40 a pair. University of Arizona, in 1963, conducting field research on In answer to the question that all young zoo enthusiasts ask: White-nosed Coatis. -

Status Report on Indonesian Galliformes
CORE Metadata, citation and similar papers at core.ac.uk Provided by KUKILA STATUS REPORT ON INDONESIAN GALLIFORMES Compiled by D.A. Holmes Introduction This is a preliminary report on the status of galliformes in Indonesia, which has been prepared with three objectives: to alert conservationists to areas of danger, to demonstrate the paucity of data for most species, and to serve as a basis for updating Information. An IUCN/ICBP Red Data Book 'category is given for each species (endangered, vulnerable, rare, Indeterminate and out of danger), but it cannot be too strongly emphasized that this category, which refers only to Indonesia, is PROVISIONAL; in many cases it 1s little more than a guess. Against each species is also stated whether or not -it is protected under Indonesian law. Direct human Intervention is believed to threaten especially the megapodes, which are heavily exploited for their eggs. Traditionally this has been done on a more or less sustainable basis, but there is accumulating evidence that any sharp increase in rural population, for example on government-sponsored transmigration schemes or spontaneous inflow of farmers from other regions, may quickly result in extermination of populations locally, especially for those megapodes that breed colonially. R. Dekker (pers. comm.) states that any one species may give an impression of being common, but if these are only adult birds, it is possible that the eggs are already being over-harvested and the population is not being replaced. Thus surveys, mapping, monitoring and management of breeding sites of certain species may be an urgent priority. Loss of habitat is the second main threat for all galliformes that are dependent on a forest habitat; this applies especially to the lowland specialists, island endemics, and those intolerant of disturbance through logging. -

Detailed Species Accounts from the Threatened Birds Of
Threatened Birds of Asia: The BirdLife International Red Data Book Editors N. J. COLLAR (Editor-in-chief), A. V. ANDREEV, S. CHAN, M. J. CROSBY, S. SUBRAMANYA and J. A. TOBIAS Maps by RUDYANTO and M. J. CROSBY Principal compilers and data contributors ■ BANGLADESH P. Thompson ■ BHUTAN R. Pradhan; C. Inskipp, T. Inskipp ■ CAMBODIA Sun Hean; C. M. Poole ■ CHINA ■ MAINLAND CHINA Zheng Guangmei; Ding Changqing, Gao Wei, Gao Yuren, Li Fulai, Liu Naifa, Ma Zhijun, the late Tan Yaokuang, Wang Qishan, Xu Weishu, Yang Lan, Yu Zhiwei, Zhang Zhengwang. ■ HONG KONG Hong Kong Bird Watching Society (BirdLife Affiliate); H. F. Cheung; F. N. Y. Lock, C. K. W. Ma, Y. T. Yu. ■ TAIWAN Wild Bird Federation of Taiwan (BirdLife Partner); L. Liu Severinghaus; Chang Chin-lung, Chiang Ming-liang, Fang Woei-horng, Ho Yi-hsian, Hwang Kwang-yin, Lin Wei-yuan, Lin Wen-horn, Lo Hung-ren, Sha Chian-chung, Yau Cheng-teh. ■ INDIA Bombay Natural History Society (BirdLife Partner Designate) and Sálim Ali Centre for Ornithology and Natural History; L. Vijayan and V. S. Vijayan; S. Balachandran, R. Bhargava, P. C. Bhattacharjee, S. Bhupathy, A. Chaudhury, P. Gole, S. A. Hussain, R. Kaul, U. Lachungpa, R. Naroji, S. Pandey, A. Pittie, V. Prakash, A. Rahmani, P. Saikia, R. Sankaran, P. Singh, R. Sugathan, Zafar-ul Islam ■ INDONESIA BirdLife International Indonesia Country Programme; Ria Saryanthi; D. Agista, S. van Balen, Y. Cahyadin, R. F. A. Grimmett, F. R. Lambert, M. Poulsen, Rudyanto, I. Setiawan, C. Trainor ■ JAPAN Wild Bird Society of Japan (BirdLife Partner); Y. Fujimaki; Y. Kanai, H. -

Polyplectron Schleiermacheri
Threatened birds of Asia BORNEAN PEACOCK-PHEASANT Polyplectron schleiermacheri Critical — Endangered C1; C2a Vulnerable A1c,d; A2c,d This elusive species’s status is difficult to judge, but recent anecdotal evidence regarding its range and habitat indicates that it has a very small, fragmented and declining population, justifying its classification as Endangered. DISTRIBUTION The Bornean Peacock-pheasant (see Remarks 1) is endemic to the island of Borneo, where it has been recorded to date from Sabah and Sarawak, Malaysia, and Kalimantan, Indonesia. The following account has drawn extensively on a compilation of important new evidence by Sözer et al. (ms a), as part of the results of their “Kalimantan Pheasant Project”, and grateful acknowledgement is made here to the authors for free access to this material. Records are from: ■ MALAYSIA ■ Sabah (see Remarks 2) Paitan river (Paitan or Paitan Bay on some labels), July and December 1892 (Gore 1968, 10 specimens in AMNH, BMNH, with three more undated ones in SMF, SNMS; see also Population); Tongod (Ulu Tongod Forest Reserve), near Telupid, January 1996 (G. W. H. Davison in litt. 1999); near the Sukau river, recently, based on a guide recognising the species from a video of Malaysian Peacock-pheasant Polyplectron malacense and describing the differences correctly (J. Corder in litt. 1999), this record being treated here as provisional; ■ Sarawak Trusan river, by report (Moulton 1914); near (on a footpath leading down from) Bario, in the late 1970s (D. Labang per P. J. K. McGowan in litt. 2000, also McGowan and Garson 1995; see Remarks 3); central Sarawak “well up toward the Dutch border” (Beebe 1918–1922), probably the upper Rejang (and hence above Belaga in Smythies 1957), in which area the local people (Punan Busang) in the Danum–Linau area at 300–1,000 m claim to know the species (Fogden 1965, Harrisson 1965, Smythies 1981, van Balen and Holmes 1993, Smythies and Davison 1999; but see Remarks 4); Nanga Gaat, c.100 km from Kapit, around 1990 (Z. -

Phasianinae Species Tree
Phasianinae Blood Pheasant,Ithaginis cruentus Ithaginini ?Western Tragopan, Tragopan melanocephalus Satyr Tragopan, Tragopan satyra Blyth’s Tragopan, Tragopan blythii Temminck’s Tragopan, Tragopan temminckii Cabot’s Tragopan, Tragopan caboti Lophophorini ?Snow Partridge, Lerwa lerwa Verreaux’s Monal-Partridge, Tetraophasis obscurus Szechenyi’s Monal-Partridge, Tetraophasis szechenyii Chinese Monal, Lophophorus lhuysii Himalayan Monal, Lophophorus impejanus Sclater’s Monal, Lophophorus sclateri Koklass Pheasant, Pucrasia macrolopha Wild Turkey, Meleagris gallopavo Ocellated Turkey, Meleagris ocellata Tetraonini Ruffed Grouse, Bonasa umbellus Hazel Grouse, Tetrastes bonasia Chinese Grouse, Tetrastes sewerzowi Greater Sage-Grouse / Sage Grouse, Centrocercus urophasianus Gunnison Sage-Grouse / Gunnison Grouse, Centrocercus minimus Dusky Grouse, Dendragapus obscurus Sooty Grouse, Dendragapus fuliginosus Sharp-tailed Grouse, Tympanuchus phasianellus Greater Prairie-Chicken, Tympanuchus cupido Lesser Prairie-Chicken, Tympanuchus pallidicinctus White-tailed Ptarmigan, Lagopus leucura Rock Ptarmigan, Lagopus muta Willow Ptarmigan / Red Grouse, Lagopus lagopus Siberian Grouse, Falcipennis falcipennis Spruce Grouse, Canachites canadensis Western Capercaillie, Tetrao urogallus Black-billed Capercaillie, Tetrao parvirostris Black Grouse, Lyrurus tetrix Caucasian Grouse, Lyrurus mlokosiewiczi Tibetan Partridge, Perdix hodgsoniae Gray Partridge, Perdix perdix Daurian Partridge, Perdix dauurica Reeves’s Pheasant, Syrmaticus reevesii Copper Pheasant, Syrmaticus -

Near Threatened Species NEAR THREATENED SPECIES
Near Threatened species NEAR THREATENED SPECIES 2483 Threatened birds of Asia DWARF CASSOWARY Casuarius bennetti occurs in New Guinea (Papua, formerly Irian Jaya, Indonesia and Papua New Guinea) and, presumably as a long-established introduction, on New Britain, where it is a forest species occurring into the mountains and occasionally to the treeline at 3,600 m (Coates 1985, Beehler et al. 1986). Although probably tolerant of moderate habitat degradation, logging opens up previously inaccessible areas to hunters; despite heavy hunting pressure, it remains relatively common over a wide altitudinal range (Coates 1985, Beehler et al. 1986, B. M. Beehler in litt. 2000, A. Mack in litt. 1999). It is judged to have a substantial population and to be declining more slowly than the other larger and more lowland cassowaries Casuarius. Criteria nearly met: A1b,d; A2b,d. PYGMY CORMORANT Phalacrocorax pygmeus breeds in (all data for pairs, and unless otherwise indicated all information from Crivelli et al. 1996) Bulgaria (20–180), Greece (1,250– 1,310) (Kazantzidis and Nazirides 1999), Italy (30–50) (M. Passarella in litt. 1999), Moldova (30–500), Hungary, Romania (4,000–7,000), Turkey (1,000–1,500) (Eken and Magnin in press), Slovakia, Yugoslavia (1,000–1,200), F.Y.R.O. Macedonia, Croatia, Ukraine (20–320), Russia, Iran (20–30), Azerbaijan (14,749 estimated in 1986), Kazakhstan, Tajikistan, Turkmenistan, and Uzebekistan, plus, possibly, south-east Iraq, and it winters primarily in Albania, Greece, Yugoslavia, F.Y.R.O. Macedonia, Turkey, Cyprus, Iraq, Iran, Azerbaijan and also Israel, Bulgaria, Romania and Syria. There is one record from Pakistan (Grimmett et al. -

Bird Checklists of the World Country Or Region: Malaysia
Avibase Page 1of 23 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Malaysia 2 Number of species: 799 3 Number of endemics: 14 4 Number of breeding endemics: 0 5 Number of introduced species: 17 6 Date last reviewed: 2020-03-19 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Malaysia. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=my [23/09/2021]. Make your observations count! Submit your data to ebird. -

Breeding and Managing Pheasants
Chapter 5 Keeping chicks alive – parent or broody reared At times, it can seem that pheasant chicks find every possible way to kill themselves or to get themselves killed. They seem to escape from the aviary through the smallest of holes, stand next to the wire dividing aviaries and get pecked by neighbouring adults or just stand out in the rain and get too wet to survive. Parent-reared chicks seem to have fewer problems but can still wander off from their mother and chill quickly. Predicting where and when problems can occur can frequently make the difference between keeping the chicks alive and losing them. The parent or broody hen provides warmth and shelter. Chicks and parents need to learn this, so limit the brooding range initially and ensure that they will not get wet if it rains. Ensure there are no obstacles where chicks can get lost – the parent can fly, but the chick can’t. Pheasants can’t count, so can easily lose chicks – however, they usually respond very rapidly to alarm calls from their chicks. Place food and water in easily accessible areas. Supply food in a shallow container where the chicks can feed easily, but remember that the parents of some species will also use this as a food source, so check that food is always available Acquire mealworms and chick crumbs before the predicted hatch date – hard boiled egg can also be very palatable and good for young chicks Provide clean water in a container that is accessible to chicks, but where they won’t get wet – pebbles make good stepping stones.