Physiological Color Change in Snakes Physiological Color Change in Snakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extreme Miniaturization of a New Amniote Vertebrate and Insights Into the Evolution of Genital Size in Chameleons
www.nature.com/scientificreports OPEN Extreme miniaturization of a new amniote vertebrate and insights into the evolution of genital size in chameleons Frank Glaw1*, Jörn Köhler2, Oliver Hawlitschek3, Fanomezana M. Ratsoavina4, Andolalao Rakotoarison4, Mark D. Scherz5 & Miguel Vences6 Evolutionary reduction of adult body size (miniaturization) has profound consequences for organismal biology and is an important subject of evolutionary research. Based on two individuals we describe a new, extremely miniaturized chameleon, which may be the world’s smallest reptile species. The male holotype of Brookesia nana sp. nov. has a snout–vent length of 13.5 mm (total length 21.6 mm) and has large, apparently fully developed hemipenes, making it apparently the smallest mature male amniote ever recorded. The female paratype measures 19.2 mm snout–vent length (total length 28.9 mm) and a micro-CT scan revealed developing eggs in the body cavity, likewise indicating sexual maturity. The new chameleon is only known from a degraded montane rainforest in northern Madagascar and might be threatened by extinction. Molecular phylogenetic analyses place it as sister to B. karchei, the largest species in the clade of miniaturized Brookesia species, for which we resurrect Evoluticauda Angel, 1942 as subgenus name. The genetic divergence of B. nana sp. nov. is rather strong (9.9‒14.9% to all other Evoluticauda species in the 16S rRNA gene). A comparative study of genital length in Malagasy chameleons revealed a tendency for the smallest chameleons to have the relatively largest hemipenes, which might be a consequence of a reversed sexual size dimorphism with males substantially smaller than females in the smallest species. -

Zootaxa, Molecular Phylogeny, Classification, and Biogeography Of
Zootaxa 2067: 1–28 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) Molecular phylogeny, classification, and biogeography of West Indian racer snakes of the Tribe Alsophiini (Squamata, Dipsadidae, Xenodontinae) S. BLAIR HEDGES1, ARNAUD COULOUX2, & NICOLAS VIDAL3,4 1Department of Biology, 208 Mueller Lab, Pennsylvania State University, University Park, PA 16802-5301 USA. E-mail: [email protected] 2Genoscope. Centre National de Séquençage, 2 rue Gaston Crémieux, CP5706, 91057 Evry Cedex, France www.genoscope.fr 3UMR 7138, Département Systématique et Evolution, Muséum National d’Histoire Naturelle, CP 26, 57 rue Cuvier, 75005 Paris, France 4Corresponding author. E-mail : [email protected] Abstract Most West Indian snakes of the family Dipsadidae belong to the Subfamily Xenodontinae and Tribe Alsophiini. As recognized here, alsophiine snakes are exclusively West Indian and comprise 43 species distributed throughout the region. These snakes are slender and typically fast-moving (active foraging), diurnal species often called racers. For the last four decades, their classification into six genera was based on a study utilizing hemipenial and external morphology and which concluded that their biogeographic history involved multiple colonizations from the mainland. Although subsequent studies have mostly disagreed with that phylogeny and taxonomy, no major changes in the classification have been proposed until now. Here we present a DNA sequence analysis of five mitochondrial genes and one nuclear gene in 35 species and subspecies of alsophiines. Our results are more consistent with geography than previous classifications based on morphology, and support a reclassification of the species of alsophiines into seven named and three new genera: Alsophis Fitzinger (Lesser Antilles), Arrhyton Günther (Cuba), Borikenophis Hedges & Vidal gen. -

The Sclerotic Ring: Evolutionary Trends in Squamates
The sclerotic ring: Evolutionary trends in squamates by Jade Atkins A Thesis Submitted to Saint Mary’s University, Halifax, Nova Scotia in Partial Fulfillment of the Requirements for the Degree of Master of Science in Applied Science July, 2014, Halifax Nova Scotia © Jade Atkins, 2014 Approved: Dr. Tamara Franz-Odendaal Supervisor Approved: Dr. Matthew Vickaryous External Examiner Approved: Dr. Tim Fedak Supervisory Committee Member Approved: Dr. Ron Russell Supervisory Committee Member Submitted: July 30, 2014 Dedication This thesis is dedicated to my family, friends, and mentors who helped me get to where I am today. Thank you. ! ii Table of Contents Title page ........................................................................................................................ i Dedication ...................................................................................................................... ii List of figures ................................................................................................................. v List of tables ................................................................................................................ vii Abstract .......................................................................................................................... x List of abbreviations and definitions ............................................................................ xi Acknowledgements .................................................................................................... -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

An Overview and Checklist of the Native and Alien Herpetofauna of the United Arab Emirates
Herpetological Conservation and Biology 5(3):529–536. Herpetological Conservation and Biology Symposium at the 6th World Congress of Herpetology. AN OVERVIEW AND CHECKLIST OF THE NATIVE AND ALIEN HERPETOFAUNA OF THE UNITED ARAB EMIRATES 1 1 2 PRITPAL S. SOORAE , MYYAS AL QUARQAZ , AND ANDREW S. GARDNER 1Environment Agency-ABU DHABI, P.O. Box 45553, Abu Dhabi, United Arab Emirates, e-mail: [email protected] 2Natural Science and Public Health, College of Arts and Sciences, Zayed University, P.O. Box 4783, Abu Dhabi, United Arab Emirates Abstract.—This paper provides an updated checklist of the United Arab Emirates (UAE) native and alien herpetofauna. The UAE, while largely a desert country with a hyper-arid climate, also has a range of more mesic habitats such as islands, mountains, and wadis. As such it has a diverse native herpetofauna of at least 72 species as follows: two amphibian species (Bufonidae), five marine turtle species (Cheloniidae [four] and Dermochelyidae [one]), 42 lizard species (Agamidae [six], Gekkonidae [19], Lacertidae [10], Scincidae [six], and Varanidae [one]), a single amphisbaenian, and 22 snake species (Leptotyphlopidae [one], Boidae [one], Colubridae [seven], Hydrophiidae [nine], and Viperidae [four]). Additionally, we recorded at least eight alien species, although only the Brahminy Blind Snake (Ramphotyplops braminus) appears to have become naturalized. We also list legislation and international conventions pertinent to the herpetofauna. Key Words.— amphibians; checklist; invasive; reptiles; United Arab Emirates INTRODUCTION (Arnold 1984, 1986; Balletto et al. 1985; Gasperetti 1988; Leviton et al. 1992; Gasperetti et al. 1993; Egan The United Arab Emirates (UAE) is a federation of 2007). -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
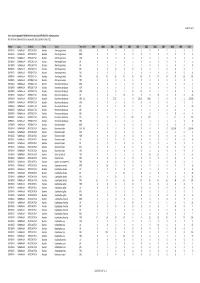
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

Calabaria and the Phytogeny of Erycine Snakes
<nological Journal of the Linnean Socieb (1993), 107: 293-351. With 19 figures Calabaria and the phylogeny of erycine snakes ARNOLD G. KLUGE Museum of <oolog~ and Department of Biology, University of Michigan, Ann Arbor, Mr 48109 U.S.A. Receiued October 1991, revised manuscript accepted Mar I992 Two major subgroups of erycine snakes, designated Charina and Eyx, are delimited with a cladistic analysis of 75 morphological characters. The hypotheses of species relationships within the two clades are (reinhardtii (bottae, triuirgata) ) and (colubrinus, conicus, elegans, jayakari, muellen’, somalicus (miliaris (tataricus (iaculus, johnii)))),respectively. This pattern of grouping obtains without assuming multistate character additivity. At least 16 synapomorphies indicate that reinhardtii is an erycine and that it is the sister lineage of the (bottae, friuirgata) cladr. Calabaria and Lichanura are synonymized with Charina for reasons of taxonomic efficiency, and to emphasize the New-Old World geographic distribution of the three species in that assemblage. Further resolution of E’yx species relationships is required before Congylophis (type species conicus) can be recognized. ADDITIONAL KEY WORDS:--Biogeography - Cladistics - erycines - fossils - taxonomy CONI‘EN’I’S Introduction ................... 293 Erycine terminal taxa and nomenclature ............ 296 Fossils .................... 301 Methods and materials ................ 302 Eryrine phylogeny ................. 306 Character descriptions ............... 306 Other variation ................ -

Species-Edition-Melanesian-Geo.Pdf
Nature Melanesian www.melanesiangeo.com Geo Tranquility 6 14 18 24 34 66 72 74 82 6 Herping the final frontier 42 Seahabitats and dugongs in the Lau Lagoon 10 Community-based response to protecting biodiversity in East 46 Herping the sunset islands Kwaio, Solomon Islands 50 Freshwater secrets Ocean 14 Leatherback turtle community monitoring 54 Freshwater hidden treasures 18 Monkey-faced bats and flying foxes 58 Choiseul Island: A biogeographic in the Western Solomon Islands stepping-stone for reptiles and amphibians of the Solomon Islands 22 The diversity and resilience of flying foxes to logging 64 Conservation Development 24 Feasibility studies for conserving 66 Chasing clouds Santa Cruz Ground-dove 72 Tetepare’s turtle rodeo and their 26 Network Building: Building a conservation effort network to meet local and national development aspirations in 74 Secrets of Tetepare Culture Western Province 76 Understanding plant & kastom 28 Local rangers undergo legal knowledge on Tetepare training 78 Grassroots approach to Marine 30 Propagation techniques for Tubi Management 34 Phantoms of the forest 82 Conservation in Solomon Islands: acts without actions 38 Choiseul Island: Protecting Mt Cover page The newly discovered Vangunu Maetambe to Kolombangara River Island endemic rat, Uromys vika. Image watershed credit: Velizar Simeonovski, Field Museum. wildernesssolomons.com WWW.MELANESIANGEO.COM | 3 Melanesian EDITORS NOTE Geo PRODUCTION TEAM Government Of Founder/Editor: Patrick Pikacha of the priority species listed in the Critical Ecosystem [email protected] Solomon Islands Hails Partnership Fund’s investment strategy for the East Assistant editor: Tamara Osborne Melanesian Islands. [email protected] Barana Community The Critical Ecosystem Partnership Fund (CEPF) Contributing editor: David Boseto [email protected] is designed to safeguard Earth’s most biologically rich Prepress layout: Patrick Pikacha Nature Park Initiative and threatened regions, known as biodiversity hotspots. -

Conservation of Amphibians and Reptiles in Indonesia: Issues and Problems
Copyright: © 2006 Iskandar and Erdelen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro- Amphibian and Reptile Conservation 4(1):60-87. duction in any medium, provided the original author and source are credited. DOI: 10.1514/journal.arc.0040016 (2329KB PDF) The authors are responsible for the facts presented in this article and for the opinions expressed there- in, which are not necessarily those of UNESCO and do not commit the Organisation. The authors note that important literature which could not be incorporated into the text has been published follow- ing the drafting of this article. Conservation of amphibians and reptiles in Indonesia: issues and problems DJOKO T. ISKANDAR1 * AND WALTER R. ERDELEN2 1School of Life Sciences and Technology, Institut Teknologi Bandung, 10, Jalan Ganesa, Bandung 40132 INDONESIA 2Assistant Director-General for Natural Sciences, UNESCO, 1, rue Miollis, 75732 Paris Cedex 15, FRANCE Abstract.—Indonesia is an archipelagic nation comprising some 17,000 islands of varying sizes and geologi- cal origins, as well as marked differences in composition of their floras and faunas. Indonesia is considered one of the megadiversity centers, both in terms of species numbers as well as endemism. According to the Biodiversity Action Plan for Indonesia, 16% of all amphibian and reptile species occur in Indonesia, a total of over 1,100 species. New research activities, launched in the last few years, indicate that these figures may be significantly higher than generally assumed. Indonesia is suspected to host the worldwide highest numbers of amphibian and reptiles species. -

Squamata: Tropidophiidae)
caribbean herpetology note Easternmost record of the Cuban Broad-banded Trope, Tropidophis feicki (Squamata: Tropidophiidae) Tomás M. Rodríguez-Cabrera1*, Javier Torres2, and Ernesto Morell Savall3 1Sociedad Cubana de Zoología, Cuba. 2 Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, Kansas 66045, USA. 3Área Protegida “Sabanas de Santa Clara,” Empresa Nacional para la Protección de la Flora y la Fauna, Villa Clara 50100, Cuba. *Corresponding author ([email protected]) Edited by: Robert W. Henderson. Date of publication: 14 May 2020. Citation: Rodríguez-Cabrera TM, Torres J, Morell Savall E (2020) Easternmost record of the Cuban Broad-banded Trope, Tropidophis feicki (Squa- mata: Tropidophiidae), of Cuba. Caribbean Herpetology, 71, 1-3. DOI: https://doi.org/10.31611/ch.71 Tropidophis feicki Schwartz, 1957 is restricted to densely forested limestone mesic areas in western Cuba (Schwartz & Henderson 1991; Henderson & Powell 2009). This species has been reported from about 20 localities distributed from near Guane, in Pinar del Río Province, to Ciénaga de Zapata, in Matanzas Province Rivalta et al., 2013; GBIF 2020; Fig. 1). On 30 June 2009 and on 22 December 2011 we found an adult male and an adult female Tropidophis feicki (ca. 400 mm SVL; Fig. 2), respectively, at the entrance of the “Cueva de la Virgen” hot cave (22.8201, -80.1384; 30 m a.s.l.; WGS 84; point 14 in Fig. 1). The cave is located within “Mogotes de Jumagua” Ecological Reserve, Sagua La Grande Municipality, Villa Clara Province. This locality represents the first record of this species for central Cuba, particularly for Villa Clara Province.