Zootaxa, Molecular Phylogeny, Classification, and Biogeography Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uromacer Catesbyi (Schlegel) 1. Uromacer Catesbyi Catesbyi Schlegel 2. Uromacer Catesbyi Cereolineatus Schwartz 3. Uromacer Cate
T 356.1 REPTILIA: SQUAMATA: SERPENTES: COLUBRIDAE UROMACER CATESBYI Catalogue of American Amphibians and Reptiles. [but see Schwartz,' 1970]); ontogenetic color change (Henderson and Binder, 1980); head and body proportions (Henderson and SCHWARTZ,ALBERTANDROBERTW. HENDERSON.1984. Uroma• Binder, 1980; Henderson et a\., 1981; Henderson, 1982b); behav• cer catesbyi. ior and ecology (Werner, 1909; Mertens, 1939; Curtiss, 1947; Uromacer catesbyi (Schlegel) Horn, 1969; Schwartz, 1970, 1979, 1980; Henderson and Binder, 1980; Henderson et a\., 1981, 1982; Henderson, 1982a, 1982b; Dendrophis catesbyi Schlegel, 1837:226. Type-locality, "lie de Henderson and Horn, 1983). St.- Domingue." Syntypes, Mus. Nat. Hist. Nat., Paris, 8670• 71 (sexes unknown) taken by Alexandre Ricord (date of col• • ETYMOLOGY.The species is named for Mark Catesby, noted lection unknown) (not examined by authors). North American naturalist. The subspecies names are all derived Uromacer catesbyi: Dumeril, Bibron, and Dumeril, 1854:72l. from Latin, as follow: cereolineatus, "waxen" and "thread," in allusion to the white longitudinal lateral line; hariolatus meaning • CONTENT.Eight subspecies are recognized, catesbyi, cereo• "predicted" in allusion to the fact that the north island (sensu lineatus,frondicolor, hariolatus, inchausteguii, insulaevaccarum, Williams, 1961) population was expected to be distinct; inchaus• pampineus, and scandax. teguii in honor of Sixto J. Inchaustegui, of the Museo Nacional de • DEFINITION.An elongate Uromacer, but head less elongate Historia Natural de Santo Domingo, Republica Dominicana; insu• than in congeners, and the head scales accordingly not highly mod• laevaccarum, a literal translation of lIe-a-Vache (island of cows), ified. Ventrals are 157-177 in males, and 155-179 in females; pampineus, "pertaining to vine tendrils or leaves;" and scandax, subcaudals are 172-208 in males, and 159-201 in females. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -
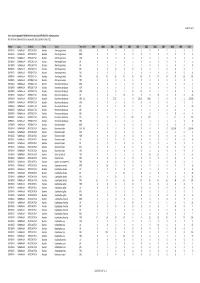
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

Ecological Functions of Neotropical Amphibians and Reptiles: a Review
Univ. Sci. 2015, Vol. 20 (2): 229-245 doi: 10.11144/Javeriana.SC20-2.efna Freely available on line REVIEW ARTICLE Ecological functions of neotropical amphibians and reptiles: a review Cortés-Gomez AM1, Ruiz-Agudelo CA2 , Valencia-Aguilar A3, Ladle RJ4 Abstract Amphibians and reptiles (herps) are the most abundant and diverse vertebrate taxa in tropical ecosystems. Nevertheless, little is known about their role in maintaining and regulating ecosystem functions and, by extension, their potential value for supporting ecosystem services. Here, we review research on the ecological functions of Neotropical herps, in different sources (the bibliographic databases, book chapters, etc.). A total of 167 Neotropical herpetology studies published over the last four decades (1970 to 2014) were reviewed, providing information on more than 100 species that contribute to at least five categories of ecological functions: i) nutrient cycling; ii) bioturbation; iii) pollination; iv) seed dispersal, and; v) energy flow through ecosystems. We emphasize the need to expand the knowledge about ecological functions in Neotropical ecosystems and the mechanisms behind these, through the study of functional traits and analysis of ecological processes. Many of these functions provide key ecosystem services, such as biological pest control, seed dispersal and water quality. By knowing and understanding the functions that perform the herps in ecosystems, management plans for cultural landscapes, restoration or recovery projects of landscapes that involve aquatic and terrestrial systems, development of comprehensive plans and detailed conservation of species and ecosystems may be structured in a more appropriate way. Besides information gaps identified in this review, this contribution explores these issues in terms of better understanding of key questions in the study of ecosystem services and biodiversity and, also, of how these services are generated. -

A Case of Envenomation by the False Fer-De-Lance Snake Leptodeira Annulata (Linnaeus, 1758) in the Department of La Guajira, Colombia
Biomédica ISSN: 0120-4157 Instituto Nacional de Salud A case of envenomation by the false fer-de-lance snake Leptodeira annulata (Linnaeus, 1758) in the department of La Guajira, Colombia Angarita-Sierra, Teddy; Montañez-Méndez, Alejandro; Toro-Sánchez, Tatiana; Rodríguez-Vargas, Ariadna A case of envenomation by the false fer-de-lance snake Leptodeira annulata (Linnaeus, 1758) in the department of La Guajira, Colombia Biomédica, vol. 40, no. 1, 2020 Instituto Nacional de Salud Available in: http://www.redalyc.org/articulo.oa?id=84362871004 DOI: 10.7705/biomedica.4773 PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Case report A case of envenomation by the false fer-de-lance snake Leptodeira annulata (Linnaeus, 1758) in the department of La Guajira, Colombia Un caso de envenenamiento por mordedura de una serpiente falsa cabeza de lanza, Leptodeira annulata (Linnaeus, 1758), en el departamento de La Guajira, Colombia Teddy Angarita-Sierra 12* Universidad Manuela Beltrán, Colombia Alejandro Montañez-Méndez 2 Fundación de Investigación en Biodiversidad y Conservación, Colombia Tatiana Toro-Sánchez 2 Fundación de Investigación en Biodiversidad y Conservación, Colombia 3 Biomédica, vol. 40, no. 1, 2020 Ariadna Rodríguez-Vargas Universidad Nacional de Colombia, Colombia Instituto Nacional de Salud Received: 17 October 2018 Revised document received: 05 August 2019 Accepted: 09 August 2019 Abstract: Envenomations by colubrid snakes in Colombia are poorly known, DOI: 10.7705/biomedica.4773 consequently, the clinical relevance of these species in snakebite accidents has been historically underestimated. Herein, we report the first case of envenomation by CC BY opisthoglyphous snakes in Colombia occurred under fieldwork conditions at the municipality of Distracción, in the department of La Guajira. -

Calabaria and the Phytogeny of Erycine Snakes
<nological Journal of the Linnean Socieb (1993), 107: 293-351. With 19 figures Calabaria and the phylogeny of erycine snakes ARNOLD G. KLUGE Museum of <oolog~ and Department of Biology, University of Michigan, Ann Arbor, Mr 48109 U.S.A. Receiued October 1991, revised manuscript accepted Mar I992 Two major subgroups of erycine snakes, designated Charina and Eyx, are delimited with a cladistic analysis of 75 morphological characters. The hypotheses of species relationships within the two clades are (reinhardtii (bottae, triuirgata) ) and (colubrinus, conicus, elegans, jayakari, muellen’, somalicus (miliaris (tataricus (iaculus, johnii)))),respectively. This pattern of grouping obtains without assuming multistate character additivity. At least 16 synapomorphies indicate that reinhardtii is an erycine and that it is the sister lineage of the (bottae, friuirgata) cladr. Calabaria and Lichanura are synonymized with Charina for reasons of taxonomic efficiency, and to emphasize the New-Old World geographic distribution of the three species in that assemblage. Further resolution of E’yx species relationships is required before Congylophis (type species conicus) can be recognized. ADDITIONAL KEY WORDS:--Biogeography - Cladistics - erycines - fossils - taxonomy CONI‘EN’I’S Introduction ................... 293 Erycine terminal taxa and nomenclature ............ 296 Fossils .................... 301 Methods and materials ................ 302 Eryrine phylogeny ................. 306 Character descriptions ............... 306 Other variation ................ -

Squamata: Tropidophiidae)
caribbean herpetology note Easternmost record of the Cuban Broad-banded Trope, Tropidophis feicki (Squamata: Tropidophiidae) Tomás M. Rodríguez-Cabrera1*, Javier Torres2, and Ernesto Morell Savall3 1Sociedad Cubana de Zoología, Cuba. 2 Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, Kansas 66045, USA. 3Área Protegida “Sabanas de Santa Clara,” Empresa Nacional para la Protección de la Flora y la Fauna, Villa Clara 50100, Cuba. *Corresponding author ([email protected]) Edited by: Robert W. Henderson. Date of publication: 14 May 2020. Citation: Rodríguez-Cabrera TM, Torres J, Morell Savall E (2020) Easternmost record of the Cuban Broad-banded Trope, Tropidophis feicki (Squa- mata: Tropidophiidae), of Cuba. Caribbean Herpetology, 71, 1-3. DOI: https://doi.org/10.31611/ch.71 Tropidophis feicki Schwartz, 1957 is restricted to densely forested limestone mesic areas in western Cuba (Schwartz & Henderson 1991; Henderson & Powell 2009). This species has been reported from about 20 localities distributed from near Guane, in Pinar del Río Province, to Ciénaga de Zapata, in Matanzas Province Rivalta et al., 2013; GBIF 2020; Fig. 1). On 30 June 2009 and on 22 December 2011 we found an adult male and an adult female Tropidophis feicki (ca. 400 mm SVL; Fig. 2), respectively, at the entrance of the “Cueva de la Virgen” hot cave (22.8201, -80.1384; 30 m a.s.l.; WGS 84; point 14 in Fig. 1). The cave is located within “Mogotes de Jumagua” Ecological Reserve, Sagua La Grande Municipality, Villa Clara Province. This locality represents the first record of this species for central Cuba, particularly for Villa Clara Province. -

Recommendations for Tourism and Biodiversity Conservation at Laguna Bavaro Wildlife Refuge, Dominican Republic
RECOMMENDATIONS FOR TOURISM AND BIODIVERSITY CONSERVATION AT LAGUNA BAVARO WILDLIFE REFUGE, DOMINICAN REPUBLIC June 2011 This publication was produced for review by the United States Agency for International Development. It was prepared in cooperation with US Forest Service, International Institute of Tropical Forestry, USAID and the Ornithological Society of Hispaniola (SOH) technical staff, and partners. Bibliographic Citation Bauer, Jerry, Jorge Brocca and Jerry Wylie. 2011. Recommendations for Tourism and Biodiversity Conservation at Laguna Bavaro Wildlife Refuge Dominican Republic. Report prepared by the US Forest Service International Institute of Tropical Forestry for the USAID/Dominican Republic in support of the Dominican Sustainable Tourism Alliance. Credits Photographs: Jerry Wylie, Jerry Bauer, Waldemar Alcobas and Jorge Brocca Graphic Design: Liliana Peralta Lopez TECHNICAL REPORT RECOMMENDATIONS FOR TOURISM AND BIODIVERSITY CONSERVATION AT LAGUNA BAVARO WILDLIFE REFUGE, DOMINICAN REPUBLIC Jerry Bauer Biological Scientist US Forest Service, International Institute of Tropical Forestry Jorge Brocca Executive Director Ornithological Society of Hispaniola and Jerry Wylie Ecotourism Specialist US Forest Service, International Institute of Tropical Forestry In cooperation with Dominican Sustainable Tourism Alliance La Altagracia Tourism Cluster Fundación Ecologica y Social Natura Park, Inc. June 2011 Sociedad Ornitológica de la Hispaniola SOH This work was completed with support from the people of the United States through USAID/Dominican Republic by the USDA Forest Service International Institute of Tropical Forestry under PAPA No. AEG-T-00-07-00003-00, TASK #7 (Sustainable Tourism Support) and the Ornithological Society of Hispaniola (SOH) in partnership with La Altagracia Tourism Cluster and assistance from local and international partners and collaborators. DISCLAIMER The authors’ views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. -

Three Rare Parrots Added to Appendix I of CITES !
PsittaScene In this Issue: Three Rare Parrots Added To Appendix I of CITES ! Truly stunning displays PPsittasitta By JAMIE GILARDI In mid-October I had the pleasure of visiting Bolivia with a group of avid parrot enthusiasts. My goal was to get some first-hand impressions of two very threatened parrots: the Red-fronted Macaw (Ara rubrogenys) and the Blue-throated Macaw (Ara SceneScene glaucogularis). We have published very little about the Red-fronted Macaw in PsittaScene,a species that is globally Endangered, and lives in the foothills of the Andes in central Bolivia. I had been told that these birds were beautiful in flight, but that Editor didn't prepare me for the truly stunning displays of colour we encountered nearly every time we saw these birds. We spent three days in their mountain home, watching them Rosemary Low, fly through the valleys, drink from the river, and eat from the trees and cornfields. Glanmor House, Hayle, Cornwall, Since we had several very gifted photographers on the trip, I thought it might make a TR27 4HB, UK stronger impression on our readers to present the trip in a collection of photos. CONTENTS Truly stunning displays................................2-3 Gold-capped Conure ....................................4-5 Great Green Macaw ....................................6-7 To fly or not to fly?......................................8-9 One man’s vision of the Trust..................10-11 Wild parrot trade: stop it! ........................12-15 Review - Australian Parrots ..........................15 PsittaNews ....................................................16 Review - Spix’s Macaw ................................17 Trade Ban Petition Latest..............................18 WPT aims and contacts ................................19 Parrots in the Wild ........................................20 Mark Stafford Below: A flock of sheep being driven Above: After tracking the Red-fronts through two afternoons, we across the Mizque River itself by a found that they were partial to one tree near a cornfield - it had sprightly gentleman. -

Molecular Authentication of Endangered Reptiles For
Molecular Authentication of Endangered Reptiles for Chinese Medicinal Materials Wong Ka Lok A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Philosophy in Biochemistry ©The Chinese University of Hong Kong July 2001 The Chinese University of Hong Kong holds the copyright of this thesis. Any person(s) intending to use a part or whole of the materials in the thesis in a proposed publication must seek copyright release from the Dean of the Graduate School. f/統系It書因 I \ UPR 12 V •F.,"- —-™ 一 � .\ �vii^W—...�/ Acknowledgements I would like to express my pleasure to my supervisor Dr RC. Shaw whc offered guidance and advice on my project. His thoughtful comments, questions and suggestions were invaluable. I also acknowledge to my other supervisor, Dr J. Wang. This work could not have been completed without the support provided by him. Special thanks goes to Mr. F.C.F. Yau for the general technical advice and support. I would like to thank the Agriculture, Fisheries and Conservation Department of HKSAR and the Kadoorie Farm & Botanic Gardens for supplying some of the samples and Environment and Conservation Fund for providing partial financial support for my work. t - i Abstract Selected DNA sequences of cytochrome b and 16S rRNA genes were amplified and sequenced from eight snake and four crocodile species. Sequence homology among these species and individuals of the same species was compared. It was found that interspecific variation was much higher than intraspecific variation and the identity of the concerned reptiles could be revealed by their DNA sequences. -

Culebra National Wildlife Refuge
Culebra National Wildlife Refuge Comprehensive Conservation Plan U.S. Department of the Interior Fish and Wildlife Service Southeast Region September 2012 COMPREHENSIVE CONSERVATION PLAN CULEBRA NATIONAL WILDLIFE REFUGE Culebra, Puerto Rico U.S. Department of the Interior Fish and Wildlife Service Southeast Region Atlanta, Georgia September 2012 Culebra National Wildlife Refuge TABLE OF CONTENTS COMPREHENSIVE CONSERVATION PLAN I. BACKGROUND ................................................................................................................................. 1 Introduction ...................................................................................................................................1 Purpose and Need for the Plan ....................................................................................................1 U.S. Fish and Wildlife Service ......................................................................................................2 National Wildlife Refuge System ..................................................................................................2 Legal and Policy Context ..............................................................................................................4 Legal Mandates, Administrative and Policy Guidelines, and Other Special Considerations .......................................................................................................4 National and International Conservation Plans and Initiatives .....................................................5