Therapeutic Applications Based on Cannabinoids Action
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cannabinoids in the Pathophysiology of Skin Inflammation
molecules Review Cannabinoids in the Pathophysiology of Skin Inflammation Cristian Scheau 1 , Ioana Anca Badarau 1, Livia-Gratiela Mihai 1, Andreea-Elena Scheau 2, Daniel Octavian Costache 3, Carolina Constantin 4,5, Daniela Calina 6 , Constantin Caruntu 1,7,*, Raluca Simona Costache 8,* and Ana Caruntu 9,10 1 Department of Physiology, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (C.S.); [email protected] (I.A.B.); [email protected] (L.-G.M.) 2 Department of Radiology and Medical Imaging, Fundeni Clinical Institute, 022328 Bucharest, Romania; [email protected] 3 Department of Dermatology, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 4 Immunology Department, ”Victor Babes” National Institute of Pathology, 050096 Bucharest, Romania; [email protected] 5 Department of Pathology, Colentina University Hospital, 020125 Bucharest, Romania 6 Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, 200349 Craiova, Romania; [email protected] 7 Department of Dermatology, “Prof. N. Paulescu” National Institute of Diabetes, Nutrition and Metabolic Diseases, 011233 Bucharest, Romania 8 Gastroenterology and Internal Medicine Clinic, Carol Davila University Central Emergency Military Hospital, Carol Davila University of Medicine and Pharmacy, 050474 Bucharest, Romania 9 Department of Oral and Maxillofacial Surgery, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 10 Faculty of Medicine, “Titu Maiorescu” University, 031593 Bucharest, Romania * Correspondence: [email protected] (C.C.); [email protected] (R.S.C.); Tel.: +40-745-086-978 (C.C.) Academic Editor: Eric J. Downer Received: 30 December 2019; Accepted: 2 February 2020; Published: 4 February 2020 Abstract: Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component. -

Nabilone for Chronic Pain Management: a Review of Clinical Effectiveness, Safety, and Guidelines
TITLE: Nabilone for Chronic Pain Management: A Review of Clinical Effectiveness, Safety, and Guidelines DATE: 11 November 2011 CONTEXT AND POLICY ISSUES Cannabis sativa is a flowering plant that has long been used as a recreational drug, and for medicinal purposes.1,2 The main psychoactive component of cannabis is delta-9- tetrahydrocannabinol (∆9-THC).2 Nabilone (Cesamet®) is an oral synthetic cannabinoid, which is licensed in Canada for treating patients with severe nausea and vomiting related to chemotherapy for cancer and who have failed to respond adequately to conventional antiemetic treatments.2-4 Clinical trials and anecdotal reports have suggested that the use of nabilone in other medical conditions, such as appetite stimulation, anxiety, spasticity, and pain.1,5 Chronic pain affects approximately one in five people in developed countries and two in five in less well-resourced countries. In many circumstances, the patient’s quality of life is poor due to persistent pain caused either by an ongoing illness or nerve damage caused by the disease after resolution or cure of the disease.6 Multiple sclerosis (MS) is a neurodegenerative disease, and is the most common cause of neurological disability in young people, with an average age of onset around 30 years and a prevalence of about 120 per 100,000 individuals in North America. The majority of patients with MS display symptoms, such as fatigue, muscle stiffness or spasticity, pain, memory problems, balance trouble, tremors, urinary disturbance, and sexual dysfunctions.2,7 The purpose of this review is to assess the evidence of benefits and harms related to the use of nabilone in management of chronic pain, including patients with MS. -
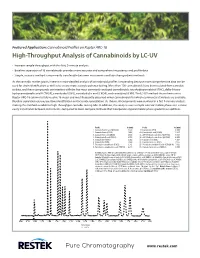
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President, -

Potential Health Benefits of Cannabis Extracts: a Review Maria Rosana Ramirez
International Journal of Chemical and Biomedical Science 2016; 2(1): 1-8 Published online March 1, 2016 (http://www.aascit.org/journal/ijcbs) Potential Health Benefits of Cannabis Extracts: A Review Maria Rosana Ramirez CITER-The National Scientific and Technical Research Council (CONICET), Argentine Government, Agency, Argentina Email address [email protected] Citation Maria Rosana Ramirez. Potential Health Benefits of Cannabis Extracts: A Review. International Journal of Chemical and Biomedical Science . Vol. 2, No. 1, 2016, pp. 1-8. Keywords Abstract Non-cannabinoids Compounds, A central tenet underlying the use of plant preparations is that herbs contain many Cannabis Extracts, bioactive compounds. Cannabis contains tetrahydrocannabinols (THC) a primary Bioactivity metabolite with reported psychotropic effects. Therefore, the presence of THC makes controversial the use of Cannabis to treat diseases by which their uses and applications were limited. The question then is: is it possible to use the extracts from Cannabis to treat the diseases related with it use in folk medicine? More recently, the synergistic Received: January 24, 2016 contributions of bioactive constituents have been scientifically demonstrated. We Revised: February 10, 2016 reviewed the literature concerning medical cannabis and its secondary metabolites, Accepted: February 12, 2016 including fraction and total extracts. Scientific evidence shows that secondary metabolites in cannabis may enhance the positive effects of THC a primary metabolite. Other chemical components (cannabinoid and non-cannabinoid) in cannabis or its extracts may reduce THC-induced anxiety, cholinergic deficits, and immunosuppression; which could increase its therapeutic potential. Particular attention will be placed on non- cannabinoid compounds interactions that could produce synergy with respect to treatment of pain, inflammation, epilepsy, fungal and bacterial infections. -

Medicinal Chemistry Endeavors Around the Phytocannabinoids
CHEMISTRY & BIODIVERSITY – Vol. 4 (2007) 1707 REVIEW Medicinal Chemistry Endeavors around the Phytocannabinoids by Eric Stern and Didier M. Lambert* Drug Design and Discovery Center and Unite´ de Chimie pharmaceutique et de Radiopharmacie, Ecole de Pharmacie, Faculte´ de Me´decine, Universite´ catholique de Louvain, Avenue E. Mounier 73, U.C.L. 73.40, B-1200 Bruxelles (phone: þ3227647347; fax: þ3227647363; e-mail: [email protected]) Over the past 50 years, a considerable research in medicinal chemistry has been carried out around the natural constituents of Cannabis sativa L. Following the identification of D9-tetrahydrocannabinol (D9-THC) in 1964, critical chemical modifications, e.g., variation of the side chain at C(3) and the opening of the tricyclic scaffold, have led to the characterization of potent and cannabinoid receptor subtype-selective ligands. Those ligands that demonstrate high affinity for the cannabinoid receptors and good biological efficacy are still used as powerful pharmacological tools. This review summarizes past as well as recent developments in the structure–activity relationships of phytocannabinoids. 1. Introduction. – Despite the wide uses of preparations of the hemp Cannabis sativa L. during the History, the modern pharmacology of natural cannabinoids has been hampered by the slow progress in the elucidations of the chemical structures of its major components. Indeed, it is nowadays known that more than 70 compounds derived from a diterpene structure are present in the plant [1], and this fact may explain the difficulty to obtain pure chemical entities in the past. In addition, the medicinal research for more than a half century has been driven by the search for the components responsible for the psychoactive effects of cannabis, this era in the history of the chemical research on cannabinoids have been recently reviewed [2][3]. -
Marinol Cannabidiol C21H30O2 Trade Name
Cannabinoids are a group of terpenophenolic compounds secreted by Cannabis flowers that provide relief from a wide array of symptoms including, pain, nausea, and inflammation. They operate by imitating the body’s natural endocannabinoids, which activate to maintain internal stability and overall health. When consumed, cannabinoids bind to receptor sites throughout the brain (CB1 receptors) and body (CB2 receptors). Different cannabinoids have different effects based on their binding affinity for each receptor. By targeting specific cannabinoids at these receptors, different types of relief can be achieved. Presently, there are at least 113 different cannabinoids isolated from Cannabis—each exhibiting varied effects. THC Tetrahydrocannabinol C21H30O2 Trade name: Marinol Legal Status: US – Schedule I, Schedule II (as Cesamet), Schedule III (as Marinol) OH CA – Schedule II UK – Class B AU – S8 (controlled) H Psychoactive Tetrahydrocannabinol (THC) is typically the most abundant cannabinoid present in cannabis products on the market today. THC has very high psychoactive characteristics and is associated with the ‘high’ and euphoria experienced when using cannabis products. When smoked or ingested, THC binds to cannabinoid receptors throughout the body and affects memory, O coordination, concentration, pleasure, and time perception. H Medicinal Benefits Analgesic • Anti-nauseant • Appetite Stimulant Reduces Glaucoma Symptoms Sleep Aid • Reduces Anxiety and PTSD Symptoms CBD Cannabidiol C21H30O2 Trade name: Epidiolex OH Legal Status: US – Schedule I CA – Schedule II UK – POM (Perscription only) H AU – S4 (Perscription only) non-psychoactive Cannabidiol (CBD) is a major phytocannabinoid and accounts for up to 40% of the plant’s extract. Due to its lack of psychoactivity and HO non-interference with motor and psychological functions, it is a leading candidate for a wide variety of medical applications. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

Federal Register/Vol. 84, No. 214/Tuesday, November 5, 2019
Federal Register / Vol. 84, No. 214 / Tuesday, November 5, 2019 / Notices 59645 Authority: 42 U.S.C. 6213; and 30 CFR company plans to bulk manufacture Controlled substance Drug Schedule 556.511–556.515. these drugs as synthetics. No other code Walter D. Cruickshank, activities for these drug codes are Hydromorphone ....................... 9150 II Acting Director, Bureau of Ocean Energy authorized for this registration. Hydrocodone ............................ 9193 II Management. Morphine .................................. 9300 II Dated: October 18, 2019. Oripavine .................................. 9330 II [FR Doc. 2019–24052 Filed 11–4–19; 8:45 am] William T. McDermott, Thebaine .................................. 9333 II BILLING CODE 4310–MR–P Assistant Administrator. Opium extracts ......................... 9610 II Opium fluid extract ................... 9620 II [FR Doc. 2019–24107 Filed 11–4–19; 8:45 am] Opium tincture ......................... 9630 II BILLING CODE 4410–09–P Opium, powdered .................... 9639 II DEPARTMENT OF JUSTICE Opium, granulated ................... 9640 II Oxymorphone .......................... 9652 II Drug Enforcement Administration Noroxymorphone ..................... 9668 II DEPARTMENT OF JUSTICE Tapentadol ............................... 9780 II [Docket No. DEA–536] Drug Enforcement Administration The company plans to manufacture Bulk Manufacturer of Controlled [Docket No. DEA–526] the listed controlled substances as an Substances Application: Organix, Inc. Active Pharmaceutical Ingredient (API) Bulk -

What Is Delta-8 THC?? Cannabinoid Chemistry 101
What is Delta-8 THC?? Cannabinoid Chemistry 101 National Conference on Weights and Measures Annual Meeting - Rochester, NY Matthew D. Curran, Ph.D. July 21, 2021 Disclaimer Just to be clear… • I am a chemist and not a lawyer so: • This presentation will not discuss the legal aspects of Δ8-THC or DEA’s current position. • This presentation will not discuss whether Δ8-THC is considered “synthetic” or “naturally occurring.” • This is not a position statement on any issues before the NCWM. • Lastly, this should only be considered a scientific sharing exercise. Florida Department of Agriculture and Consumer Services 2 Cannabis in Florida Cannabis Syllabus • What is Cannabis? • “Mother” Cannabinoid • Decarboxylation • Relationship between CBD and THC • What does “Total” mean? • Dry Weight vs. Wet Weight • What does “Delta-9” mean? • Relationship between “Delta-8” and “Delta-9” • CBD to Delta-8 THC • Cannabinoid Chemistry 202… Florida Department of Agriculture and Consumer Services 3 Cannabis Cannabis • Cannabis sativa is the taxonomic name for the plant. • The concentration of Total Δ9-Tetrahydrocannabinol (Total Δ9-THC) is critical when considering the varieties of Cannabis sativa. • Hemp – (Total Δ9-THC) 0.3% or less • Not really a controversial term, “hemp” • Marijuana/cannabis – (Total Δ9-THC) Greater than 0.3% • Controversial term, “marijuana” • Some states prohibit the use of this term whereas some states have it in their laws. • Some states use the term “cannabis.” • Not italicized • Lower case “c” Florida Department of Agriculture and -

2 Spice English Presentation
Spice Spice contains no compensatory substances Специи не содержит компенсационные вещества Spice is a mix of herbs (shredded plant material) and manmade chemicals with mind-altering effects. It is often called “synthetic marijuana” because some of the chemicals in it are similar to ones in marijuana; but its effects are sometimes very different from marijuana, and frequently much stronger. It is most often labeled “Not for Human Consumption” and disguised as incense. Eliminationprocess • The synthetic agonists such as THC is fat soluble. • Probably, they are stored as THC in cell membranes. • Some of the chemicals in Spice, however, attach to those receptors more strongly than THC, which could lead to a much stronger and more unpredictable effect. • Additionally, there are many chemicals that remain unidentified in products sold as Spice and it is therefore not clear how they may affect the user. • Moreover, these chemicals are often being changed as the makers of Spice alter them to avoid the products being illegal. • To dissolve the Spice crystals Acetone is used endocannabinoids synhtetic THC cannabinoids CB1 and CB2 agonister Binds to cannabinoidreceptor CB1 CB2 - In the brain -in the immune system Decreased avtivity in the cell ____________________ Maria Ellgren Since some of the compounds have a longer toxic effects compared to naturally THC, as reported: • negative effects that often occur the day after consumption, as a general hangover , but without nausea, mentally slow, confused, distracted, impairment of long and short term memory • Other reports mention the qualitative impairment of cognitive processes and emotional functioning, like all the oxygen leaves the brain. -

In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa
EDUCATUM JSMT Vol. 4 No. 2 (2017) ISSN 2289-7070 / eISSN 2462-2451 (1-7) https://ejournal.upsi.edu.my/journal/EDSC In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa Shakinaz Desa1*, Asiah Osman2, and Richard Hyslop3 1Department of Biology, Universiti Pendidikan Sultan Idris, Malaysia, 2Natural Product Division, Forest Research Institute Malaysia, 3Department of Chemistry and Biochemistry, University of Northern Colorado, USA *Corresponding author: [email protected] Abstract This study investigated drug-like properties of phytocannabinoids in Cannabis sativa using an in silico study. We report sixteen phytocannabinoids: cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), cannabicyclol (CBL), cannabivarin (CBV), cannabidivarin (CBDV), cannabichromevarin (CBCV), cannabigerovarin (CBGV), cannabinodiol (CBDL), cannabielsoin (CBE), cannabitriol (CBT), Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabivarin (Δ9-THCV), and Δ8-tetrahydrocannabinol (Δ8-THC). All chemical structures and properties were obtained from PubChem Compound, National Center for Biotechnology Information, U.S. National Library of Medicine. Molinspiration was used for the calculation of molecular properties and bioactivity score. The parameters were molecular weight (MW), number of hydrogen acceptor (HBA), number of hydrogen donor (HBD), partition coefficient (cLogP), polar surface area (PSA) and number of rotatable bonds (NROTB). We predicted bioactivity scores for G Protein-Coupled Receptors (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. Lipinski’s rule was used as reference to determine the drug-like properties of the phytocannabinoids. All compounds have MW<500, HBA<10, HBD<5, TPSA<140Å2 and NRTOB<10. Bioactivity score showed an active or moderately active in all compounds.