The Intraocular Changes of Anterior Segment Necrosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

The Distribution of Immune Cells in the Uveal Tract of the Normal Eye
THE DISTRIBUTION OF IMMUNE CELLS IN THE UVEAL TRACT OF THE NORMAL EYE PAUL G. McMENAMIN Perth, Western Australia SUMMARY function of these cells in the normal iris, ciliary body Inflammatory and immune-mediated diseases of the and choroid. The role of such cell types in ocular eye are not purely the consequence of infiltrating inflammation, which will be discussed by other inflammatory cells but may be initiated or propagated authors in this issue, is not the major focus of this by immune cells which are resident or trafficking review; however, a few issues will be briefly through the normal eye. The uveal tract in particular considered where appropriate. is the major site of many such cells, including resident tissue macro phages, dendritic cells and mast cells. This MACRO PHAGES review considers the distribution and location of these and other cells in the iris, ciliary body and choroid in Mononuclear phagocytes arise from bone marrow the normal eye. The uveal tract contains rich networks precursors and after a brief journey in the blood as of both resident macrophages and MHe class 11+ monocytes immigrate into tissues to become macro dendritic cells. The latter appear strategically located to phages. In their mature form they are widely act as sentinels for capturing and sampling blood-borne distributed throughout the body. Macrophages are and intraocular antigens. Large numbers of mast cells professional phagocytes and play a pivotal role as are present in the choroid of most species but are effector cells in cell-mediated immunity and inflam virtually absent from the anterior uvea in many mation.1 In addition, due to their active secretion of a laboratory animals; however, the human iris does range of important biologically active molecules such contain mast cells. -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -

Ciliary Zonule Sclera (Suspensory Choroid Ligament)
ACTIVITIES Complete Diagrams PNS 18 and 19 Complete PNS 23 Worksheet 3 #1 only Complete PNS 24 Practice Quiz THE SPECIAL SENSES Introduction Vision RECEPTORS Structures designed to respond to stimuli Variable complexity GENERAL PROPERTIES OF RECEPTORS Transducers Receptor potential Generator potential GENERAL PROPERTIES OF RECEPTORS Stimulus causing receptor potentials Generator potential in afferent neuron Nerve impulse SENSATION AND PERCEPTION Stimulatory input Conscious level = perception Awareness = sensation GENERAL PROPERTIES OF RECEPTORS Information conveyed by receptors . Modality . Location . Intensity . Duration ADAPTATION Reduction in rate of impulse transmission when stimulus is prolonged CLASSIFICATION OF RECEPTORS Stimulus Modality . Chemoreceptors . Thermoreceptors . Nociceptors . Mechanoreceptors . Photoreceptors CLASSIFICATION OF RECEPTORS Origin of stimuli . Exteroceptors . Interoceptors . Proprioceptors SPECIAL SENSES Vision Hearing Olfaction Gustation VISION INTRODUCTION 70% of all sensory receptors are in the eye Nearly half of the cerebral cortex is involved in processing visual information Optic nerve is one of body’s largest nerve tracts VISION INTRODUCTION The eye is a photoreceptor organ Refraction Conversion (transduction) of light into AP’s Information is interpreted in cerebral cortex Eyebrow Eyelid Eyelashes Site where conjunctiva merges with cornea Palpebral fissure Lateral commissure Eyelid Medial commissure (a) Surface anatomy of the right eye Figure 15.1a Orbicularis oculi muscle -

Affections of Uvea Affections of Uvea
AFFECTIONS OF UVEA AFFECTIONS OF UVEA Anatomy and physiology: • Uvea is the vascular coat of the eye lying beneath the sclera. • It consists of the uvea and uveal tract. • It consists of 3 parts: Iris, the anterior portion; Ciliary body, the middle part; Choroid, the third and the posterior most part. • All the parts of uvea are intimately associated. Iris • It is spongy having the connective tissue stroma, muscular fibers and abundance of vessels and nerves. • It is lined anteriorly by endothelium and posteriorly by a pigmented epithelium. • Its color is because of amount of melanin pigment. Mostly it is brown or golden yellow. • Iris has two muscles; the sphincter which encircles the pupil and has parasympathetic innervation; the dilator which extends from near the sphincter and has sympathetic innervation. • Iris regulates the amount of light admitted to the interior through pupil. • The iris separates the anterior chamber from the posterior chamber of the eye. Ciliary Body: • It extends backward from the base of the iris to the anterior part of the choroid. • It has ciliary muscle and the ciliary processes (70 to 80 in number) which are covered by ciliary epithelium. Choroid: • It is located between the sclera and the retina. • It extends from the ciliaris retinae to the opening of the optic nerve. • It is composed mainly of blood vessels and the pigmented tissue., The pupil • It is circular and regular opening formed by the iris and is larger in dogs in comparison to man. • It contracts or dilates depending upon the light source, due the sphincter and dilator muscles of the iris, respectively. -
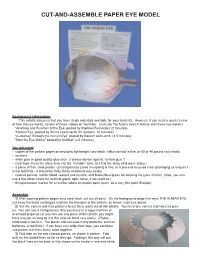
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

Glaucoma Is One of the Leading Causes of Blindness in Animals and People
www.southpaws.com SouthPaws Ophthalmology Service 8500 Arlington Boulevard Fairfax, Virginia 22031 Tel: 703.752.9100 Fax: 703.752.9200 Glaucoma is one of the leading causes of blindness in animals and people. Glaucoma is defined as increased pressure within the eye (greater than 25 mmHg), beyond that which is compatible with normal ocular function and vision. It is caused by a disturbance in the flow of fluid within and out of the globe. Primary glaucoma occurs when there are no other underlying problems causing the pressure elevation and is usually inherited. Secondary glaucoma occurs when there are other problems within the eye which have contributed to the elevated pressure. Examples of these underlying problems include uveitis (inflammation) and luxation (shifting from the normal position) of the lens. Glaucoma The fluid that fills the eye (the aqueous humour) is produced by the ciliary body, a structure which is located behind the iris and circles for 360 degrees around the eye. The fluid produced by the ciliary body flows through the pupil and fills the anterior chamber. Normally, this fluid flows out from the eye through the iridocorneal drainage angle, a structure encircling the eye where the iris and cornea meet. In a normal eye the fluid production and outflow are evenly matched, and this keeps the intraocular pressure steady. Glaucoma occurs when there is an obstruction to the outflow of the fluid which causes the fluid to build up and increase the intraocular pressure. As the pressure increases, several changes can occur within the eye: 1) Increased pressure forces fluid into the cornea and disrupts the arrangement of the protein fibers that compose the cornea. -

The Proteomes of the Human Eye, a Highly Compartmentalized Organ
Proteomics 17, 1–2, 2017, 1600340 DOI 10.1002/pmic.201600340 (1 of 3) 1600340 The proteomes of the human eye, a highly compartmentalized organ Gilbert S. Omenn Center for Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, MI, USA Proteomics has now published a series of Dataset Briefs on the EyeOme from the HUPO Received: November 2, 2016 Human Proteome Project with high-quality analyses of the proteomes of these compartments Accepted: November 4, 2016 of the human eye: retina, iris, ciliary body, retinal pigment epithelium/choroid, retrobulbar optic nerve, and sclera, with 3436, 2929, 2867, 2755, 2711, and 1945 proteins, respectively. These proteomics resources represent a useful starting point for a broad range of research aimed at developing preventive and therapeutic interventions for the various causes of blindness. Keywords: Biomedicine / Biology and Disease-driven Human Proteome Project / End Blindness by 2020 / Eye proteome / EyeOme / Human Proteome Project See accompanying articles in the EyeOme series: http://dx.doi.org/10.1002/pmic.201600229; http://dx.doi.org/10.1002/pmic.201500188; http://dx.doi.org/10.1002/pmic.201400397 Proteomics has now published a series of four papers on compartments of the eye as shown in Fig. 1. As was noted [5], the human eye proteome [1–4]. Under the aegis of the Hu- it was not feasible to assess the quality of the data or estimate man Proteome Organization Biology and Disease-driven Hu- numbers of likely false positives in the heterogeneous studies man Proteome Project (HPP), the EyeOme was organized by from which these findings were summarized. -

The Behavior of Fibroblasts from the Developing Avian Cornea
THE BEHAVIOR OF FIBROBLASTS FROM THE DEVELOPING AVIAN CORNEA Morphology and Movement In Situ and In Vitro JONATHAN B. L. BARD and ELIZABETH D. HAY From the Department of Anatomy, Harvard Medical School, Boston, Massachusetts 02115. Dr. Bard's present address is the Medical Research Council Unit, Western General Hospital, Edinburgh 4, Scotland. ABSTRACT The early chick cornea is composed of an acellular collagenous stroma lined with an anterior epithelium and a posterior endothelium. At stage 2?-28 of development (51/2 days), this stroma swells so that the cornea is 75 120 #m thick. At the same time, fibroblasts that originate from the neural crest begin to invade this stroma. Using Nomarski light microscopy, we have compared the behavior of moving cells in isolated corneas with the migratory activities of the same cells in artificial collagen lattices and on glass. In situ, fibroblasts have cyclindrical bodies from which extend several thick pseudopodia and/or finer filopodia. Movement is accompanied by activity in these cytoplasmic processes. The flat ruffling lamelli- podia that characterize these cells on glass are not seen in situ, but the general mechanism of cell movement seems to be the same as that observed in vitro: either gross contraction or recoil of the cell body (now pear shaped) into the forward cell process, or more subtle "flowing" of cytoplasm into the forward cell process without immediate loss of the trailing cell process. We filmed collisions between cells in situ and in three-dimensional collagen lattices. These fibroblasts show, in their pair-wise collisions, the classical contact inhibition of movement (CIM) ex- hibited in vitro even though they lack ruffled borders. -

Scleral Lenses and Eye Health
Scleral Lenses and Eye Health Anatomy and Function of the Human Eye How Scleral Lenses Interact with the Ocular Surface Just as the skin protects the human body, the ocular surface protects the human Scleral lenses are large-diameter lenses designed to vault the cornea and rest on the conjunctival tissue sitting on eye. The ocular surface is made up of the cornea, the conjunctiva, the tear film, top of the sclera. The space between the back surface of the lens and the cornea acts as a fluid reservoir. Scleral and the glands that produce tears, oils, and mucus in the tear film. lenses can range in size from 13mm to 19mm, although larger diameter lenses may be designed for patients with more severe eye conditions. Due to their size, scleral lenses consist SCLERA: The sclera is the white outer wall of the eye. It is SCLERAL LENS made of collagen fibers that are arranged for strength rather of at least two zones: than transmission of light. OPTIC ZONE The optic zone vaults over the cornea CORNEA: The cornea is the front center portion of the outer Cross section of FLUID RESERVOIR wall of the eye. It is made of collagen fibers that are arranged in the eye shows The haptic zone rests on the conjunctiva such a way so that the cornea is clear. The cornea bends light the cornea, overlying the sclera as it enters the eye so that the light is focused on the retina. conjunctiva, and sclera as CORNEA The cornea has a protective surface layer called the epithelium. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Pupil Iris Ciliary Body
Eye iris pupil ciliary body Eyeball Anterior segment Posterior segment Pars caeca retinae Eyeball Corpus ciliare Procesus ciliares Sclera Iris Cornea Eyebulb wall Posterior Anterior segment segment Tunica externa Sclera Cornea (fibrosa) Tunica media Chorioidea Iris, Corpus (vasculosa) ciliare Tunica interna Pars optica Pars caeca (nervosa) retinae retinae Eyeball Fibrous tunic - tunica externa oculi • Cornea • Sclera limbus conjunctiva Cornea 1. Stratified squamous epithelium 2. Bowman´s membrane-anterior limiting lamina 3. Substantia propria cornae − 200 - 250 layers of regularly organized collagen fibrils − fibrocytes /keratocytes/ 4. Descemet´s membrane-posterior limiting lamina − the basement membrane of the posterior endothelium − Posterior endothelium − simple squamous epithelium Cornea Vascular tunic - tunica media oculi - Choroid ch - loose c.t. with network of blood vessels, numerous pigment cells c - Ciliary body - loose c.t. with smooth muscle cells – musculus i ciliaris /accomodation/ - ciliary processes – generate aqueous humor - Iris - central opening of the iris - the pupil Choroid 1. Lamina suprachoroidea /lamina fusca sclerae/ 2. Lamina vasculosa 3. Lamina chorocapillaris 4. Lamina vitrea /Bruch´s membrane/ L. suprachoroidea - perichoroidal space with melanocytes, collagen and elastic sclera fibers, fibroblasts, macrophages, lymphocytes L. vasculosa – blood supply – parallelní veins – c. ciliare choroid L. chorocapilaris – capillary plexus for retina L. vitrea – five layers, including balsal retina lamina of chorid endothelium and retinal pigment epithelium, collagen and elastic fibers. Choroid Ciliary body - structure ciliary processes m. ciliaris ciliary epithelium - outer cell layer is pigmented, inner cell layer is nonpigmented (pars caeca retinae) Ciliary body Epithelium two layers – basal, pigmented (fuscin), surface w/o pigment - Continuous with optical part of retina = pars caeca retinae processus ciliares m.