Multimodality Imaging of Pericardial Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Comparative Cardiac Anatomy
5 Comparative Cardiac Anatomy ALEXANDER J. HILL, PhD AND PAUL A. IAIZZO, PhD CONTENTS HISTORICAL PERSPECTIVE OF ANATOMY AND ANIMAL RESEARCH IMPORTANCE OF ANATOMY AND ANIMAL RESEARCH LARGE MAMMALIANCOMPARATIVE CARDIAC ANATOMY REFERENCES 1. HISTORICAL PERSPECTIVE the dominion of man and, although worthy of respect, could be OF ANATOMY AND ANIMAL RESEARCH used to obtain information if it was for a "higher" purpose (2). Anatomy is one of the oldest branches of medicine, with his- Descartes described humans and other animals as complex torical records dating back at least as far as the 3rd century BC; machines, with the human soul distinguishing humans from animal research dates back equally as far. Aristotle (384-322 BC) all other animals. This beast-machine concept was important studied comparative animal anatomy and physiology, and for early animal researchers because, if animals had no souls, Erasistratus of Ceos (304-258 BC) studied live animal anatomy it was thought that they could not suffer pain. Furthermore, the and physiology (1). Galen of Pergamum (129-199 AD) is prob- reactions of animals were thought to be the response of auto- ably the most notable early anatomist who used animals in mata and not reactions of pain (2). research to attempt to understand the normal structure and The concept of functional biomedical studies can probably function of the body (2). He continuously stressed the cen- be attributed to another great scientist and anatomist, William trality of anatomy and made an attempt to dissect every day Harvey (1578-1657 AD). He is credited with one of the most because he felt it was critical to learning (3). -

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Chapter Xi the Circulatory System and Blood
CHAPTER XI THE CIRCULATORY SYSTEM AND BLOOD Page General characterlstlcs______ __ __ _ __ __ __ __ __ __ _ 239 of these organs are independent of the beating of The pericardium ___ __ __ __ 239 the principal heart, and their primary function is The heart. _____ __ __ 240 Physiology of the heart.______________________________________________ 242 to oscillate the blood within the pallial sinuses. Automatism of heart beat. _ 242 The pacemaker system_ 245 THE PERICARDIUM Methods of study of heart beat_____________________________________ 247 Frequency of beat___ __ __ _ 248 Extracardlac regulatlon____ __ __ _ 250 The heart is located in the pericardium, a thin Effects of mineral salts and drugs___________________________________ 251 Blood vessels_ __ ___ _ 253 walled chamber between the visceral mass and the The arterial system______ __ __ ___ __ __ __ __ __ 253 adductor muscle (fig. 71). In a live oyster the The venous system_________________________________________________ 254 location of the heart is indicated by the throbbing The accessory heart._____________ 258 The blood______ __ __ __ __ __ __ __ 259 of the wall of the pericardium on the left side. Color of blood_ __ __ 261 Here the pericardium wall lies directly under the The hyaline cells___________________________________________________ 261 The granular cells .______________________________________ 262 shell. On the right side the promyal chamber Specific gravity of blood____________________________________________ 265 extends down over the heart region and the mantle Serology ___ __________ __________________ ____ __ ______________________ 265 Bibliography __ __ __ __ __ __ __ 266 separates the pericardium wall from the shell. The cavity in which the heart is lodged is slightly A heart, arteries, veins, and open sinuses form asymmetrical; on the right side it extends farther the circulatory system of oysters and other bi along the anterior part of the adductor muscle valves. -

Resting Coronary Flow Velocity in The
European Heart Journal – Cardiovascular Imaging (2012) 13,79–85 CLINICAL/ORIGINAL PAPERS doi:10.1093/ehjci/jer153 Resting coronary flow velocity in the functional evaluation of coronary artery stenosis: study on sequential use of computed tomography angiography and transthoracic Doppler Downloaded from https://academic.oup.com/ehjcimaging/article/13/1/79/2397059 by guest on 30 September 2021 echocardiography Esa Joutsiniemi 1, Antti Saraste 1,2*, Mikko Pietila¨ 1, Heikki Ukkonen 1,2, Sami Kajander 2, Maija Ma¨ki 2,3, Juha Koskenvuo 3, Juhani Airaksinen 1, Jaakko Hartiala 3, Markku Saraste 3, and Juhani Knuuti 2 1Department of Cardiology, Turku University Hospital, Kiinamyllynkatu 4-8, 20520 Turku, Finland; 2Turku PET Centre, Kiinamyllynkatu 4-8, 20520 Turku, Finland; and 3Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Kiinamyllynkatu 4-8, 20520 Turku, Finland Received 10 March 2011; accepted after revision 2 August 2011; online publish-ahead-of-print 30 August 2011 Aims Accelerated flow at the site of flow-limiting stenosis can be detected by transthoracic Doppler echocardiography (TTDE). We studied feasibility and accuracy of sequential coronary computed tomography angiography (CTA) and TTDE in detection of haemodynamically significant coronary artery disease (CAD). ..................................................................................................................................................................................... Methods We prospectively enrolled 107 patients with intermediate (30–70%) pre-test likelihood of CAD. All patients under- and results went CTA using a 64-slice scanner. Using TTDE, the ratio of maximal diastolic flow velocity to pre-stenotic flow velocity (M/P ratio) was measured in the coronary segments with stenosis in CTA. In all patients, the results were compared with invasive coronary angiography, including measurement of fractional flow reserve when appropriate. -

Physiology H Digestive
2/28/18 Introduction • Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends Digestive System at the anus • Digestive functions are initiated by the parasympathetic division Chapter 29 - Digestion occurs during periods of low activity - Produces more energy than it uses Copyright © 2016 by Elsevier Inc. All rights reserved. 1 Copyright © 2016 by Elsevier Inc. All rights reserved. 2 Anatomy The Digestive System • Oral cavity • Pharynx • Esophagus • Stomach • Small intestine and large intestine • Accessory organs: Pancreas, liver, and gallbladder From Herlihy B: The human body in health and illness, ed 4, St. Louis, 2011, Saunders. Copyright © 2016 by Elsevier Inc. All rights reserved. 3 Copyright © 2016 by Elsevier Inc. All rights reserved. 4 Physiology Gastrointestinal Tract • Ingestion: Taking materials into mouth by • Muscular tube throughout digestive system eating/drinking • Accessory organs and glands secrete • Digestion: Breaking down food into molecules substance to aid in digestion that can be used by the body • GI tract wall has four layers: - Includes mechanical and enzymatic action - Mucosa • Absorption: Simple molecules from the - Submucosa gastrointestinal (GI) tract move into the - Muscle layer: Responsible for peristalsis bloodstream or lymph vessels and then into - Serosa body cells • Defecation: Eliminating indigestible or unabsorbed material from the body Copyright © 2016 by Elsevier Inc. All rights reserved. 5 Copyright © 2016 by Elsevier Inc. All rights reserved. 6 1 2/28/18 Peristalsis Oral Cavity • First portion of GI tract • Contains: - Teeth - Tongue - Openings for salivary glands From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St. -

Myocarditis, Pericarditis and Other Pericardial Diseases
Heart 2000;84:449–454 Diagnosis is easiest during epidemics of cox- GENERAL CARDIOLOGY sackie infections but diYcult in isolated cases. Heart: first published as 10.1136/heart.84.4.449 on 1 October 2000. Downloaded from These are not seen by cardiologists unless they develop arrhythmia, collapse or suVer chest Myocarditis, pericarditis and other pain, the majority being dealt with in the primary care system. pericardial diseases Acute onset of chest pain is usual and may mimic myocardial infarction or be associated 449 Celia M Oakley with pericarditis. Arrhythmias or conduction Imperial College School of Medicine, Hammersmith Hospital, disturbances may be life threatening despite London, UK only mild focal injury, whereas more wide- spread inflammation is necessary before car- diac dysfunction is suYcient to cause symp- his article discusses the diagnosis and toms. management of myocarditis and peri- Tcarditis (both acute and recurrent), as Investigations well as other pericardial diseases. The ECG may show sinus tachycardia, focal or generalised abnormality, ST segment eleva- tion, fascicular blocks or atrioventricular con- Myocarditis duction disturbances. Although the ECG abnormalities are non-specific, the ECG has Myocarditis is the term used to indicate acute the virtue of drawing attention to the heart and infective, toxic or autoimmune inflammation of leading to echocardiographic and other investi- the heart. Reversible toxic myocarditis occurs gations. Echocardiography may reveal segmen- in diphtheria and sometimes in infective endo- -
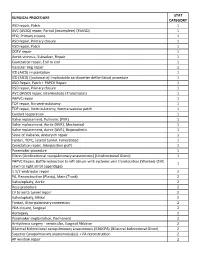
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Download PDF File
Folia Morphol. Vol. 78, No. 2, pp. 283–289 DOI: 10.5603/FM.a2018.0077 O R I G I N A L A R T I C L E Copyright © 2019 Via Medica ISSN 0015–5659 journals.viamedica.pl Observations of foetal heart veins draining directly into the left and right atria J.H. Kim1, O.H. Chai1, C.H. Song1, Z.W. Jin2, G. Murakami3, H. Abe4 1Department of Anatomy and Institute of Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea 2Department of Anatomy, Wuxi Medical School, Jiangnan University, Wuxi, China 3Division of Internal Medicine, Jikou-kai Clinic of Home Visits, Sapporo, Japan 4Department of Anatomy, Akita University School of Medicine, Akita, Japan [Received: 19 June 2018; Accepted: 8 August 2018] Evaluation of semiserial sections of 14 normal hearts from human foetuses of gestational age 25–33 weeks showed that all of these hearts contained thin veins draining directly into the atria (maximum, 10 veins per heart). Of the 75 veins in these 14 hearts, 55 emptied into the right atrium and 20 into the left atrium. These veins were not accompanied by nerves, in contrast to tributaries of the great cardiac vein, and were negative for both smooth muscle actin (SMA) and CD34. However, the epithelium and venous wall of the anterior cardiac vein, the thickest of the direct draining veins, were strongly positive for SMA and CD34, respectively. In general, developing fibres in the vascular wall were positive for CD34, while the endothelium of the arteries and veins was strongly positive for the present DAKO antibody of SMA. -

Pericardial Effusion
Pericardial Effusion ABOUT THE DIAGNOSIS are incurable, and treatment is designed to extend life and keep Pericardial effusion refers to an accumulation of fluid around the heart, the pet comfortable. Other underlying causes may be correctable, within the pericardium. The pericardium is a membranous sac that such as foreign bodies or coagulation disorders. surrounds the heart. When fluid accumulates slowly, the pericardium stretches and enlarges to accommodate the fluid, meaning that symp- TREATMENT toms are absent or delayed. A more rapid accumulation can cause If cardiac tamponade is present, the fluid must be drained promptly immediate symptoms, even with relatively small amounts of pericardial by a procedure called pericardiocentesis. Using local anesthetic, your fluid accumulation. The presence of fluid causes symptoms because veterinarian passes a catheter between the ribs into the pericardial the fluid compresses the heart and interferes with normal filling of the sac, and the fluid is drawn off. Alleviating the fluid accumulation that heart with blood. Less blood filling the heart means that less blood compresses the heart will rapidly stabilize a pet’s circulation and is pumped to the body with each heartbeat. Pericardial effusion can cardiovascular status in the vast majority of cases. Treatment then increase the external pressure on the heart to the point that delivery of depends upon the cause of the condition. If the underlying condition blood to the body is severely compromised, a condition called cardiac cannot be corrected, sometimes a procedure called pericardiectomy tamponade. Severe cardiac tamponade is a life-threatening condition. is performed. This is a surgery of the chest in which the pericardial Pericardial effusion is more common in older, large breed dogs. -

1 SIZE, FORM, and LOCATION of the HEART FIGURE 20.2A 1. The
SIZE, FORM, AND LOCATION OF THE HEART FIGURE 20.2a 1. The heart is a cone-shaped structure that is approximately the size of a fist. 2. The apex (tip) of the heart points inferiorly, and the base of the heart is superior. Thus the cone is upside down. 3. The heart is part of the mediastinum, a partition of organs that divides the thoracic cavity into right and left halves. ANATOMY OF THE HEART Pericardium FIGURE 20.3 1. The heart is enclosed by a double-layered sac called the pericardium, or the pericardial sac. The pericardium has two main parts. A. The outer fibrous pericardium consists of connective tissue and is responsible for the strength of the pericardium. B. The inner serous pericardium is simple squamous epithelium that secretes serous fluid. It protects the heart against friction. The serous pericardium has two parts. 1) The visceral pericardium is in contact with the heart. 2) The parietal pericardium is in contact with the fibrous pericardium. 3) The pericardial cavity in-between the visceral and parietal pericardium is filled with pericardial fluid. 2. Pericarditis is inflammation of the pericardium that can cause substernal pain. It can be caused by infections or damage caused by radiation treatment for cancer. 3. Cardiac tamponade [tam-po-nAd′] is compression of the heart due to an accumulation of fluid in the pericardial space. Decreased venous input results in decreased output and death can result. Cardiac tamponade can be caused by pericarditis (build up of pericardial fluid) or wounds, such as stab or gunshot wounds (buildup of blood). -

The Challenge of Assessing Heart Valve Prostheses by Doppler Echocardiography
Editorial Comment The Challenge of Assessing Heart Valve Prostheses by Doppler Echocardiography Helmut Baumgartner, MD, Muenster, Germany The assessment of prosthetic valve function remains challenging. by continuous-wave Doppler measurement. Using these velocities Echocardiography has become the key diagnostic tool not only be- for the calculation of transvalvular gradients results in marked overes- cause of its noninvasive nature and wide availability but also because timation of the actual pressure drop across the prostheses.6 The fact of limitations inherent in alternative diagnostic techniques. Invasive that this phenomenon more or less disappears in malfunctioning bi- evaluation is limited particularly in mechanical valves that cannot leaflet prostheses when the funnel-shaped central flow channel ceases be crossed with a catheter, and in patients with both aortic and mitral to exist because of restricted leaflet motion makes the interpretation valve replacements, full hemodynamic assessment would even of Doppler data and their use for accurate detection of prosthesis mal- require left ventricular puncture. Although fluoroscopy and more function even more complicated.7 Furthermore, the lack of a flat ve- recently computed tomography allow the visualization of mechanical locity profile and the central high velocities described above cause valves and the motion of their occluders, the evaluation of prosthetic erroneous calculations of valve areas when the continuity equation valves typically relies on Doppler echocardiography. incorporates such measurements.8 For these reasons, the analysis of Although Doppler echocardiography has become an ideal nonin- occluder motion using fluoroscopy (in mitral prostheses, this may vasive technique for the evaluation of native heart valves and their also be obtained on transesophageal echocardiography) remains es- function, the assessment of prosthetic valves has remained more dif- sential to avoid the misinterpretation of high Doppler velocities across ficult. -

Location of the Human Sinus Node in Black Africans
ogy: iol Cu ys r h re P n t & R y e s Anatomy & Physiology: Current m e o a t r a c n h A Research Meneas et al., Anat Physiol 2017, 7:5 ISSN: 2161-0940 DOI: 10.4172/2161-0940.1000279 Research article Open Access Location of the Human Sinus Node in Black Africans Meneas GC*, Yangni-Angate KH, Abro S and Adoubi KA Department of Cardiovascular and Thoracic Diseases, Bouake Teaching Hospital, Cote d’Ivoire, West-Africa *Corresponding author: Meneas GC, Department of Cardiovascular and Thoracic Diseases, Bouake Teaching Hospital, Cote d’Ivoire, West-Africa, Tel: +22507701532; E-mail: [email protected] Received Date: August 15, 2017; Accepted Date: August 22, 2017; Published Date: August 29, 2017 Copyright: © 2017 Meneas GC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Abstract Objective: The purpose of this study was to describe, in 45 normal hearts of black Africans adults, the location of the sinoatrial node. Methods: After naked eye observation of the external epicardial area of the sinus node classically described as cavoatrial junction (CAJ), a histological study of the sinus node area was performed. Results: This study concluded that the sinus node is indistinguishable to the naked eye (97.77% of cases), but still identified histologically at the CAJ in the form of a cluster of nodal cells surrounded by abundant connective tissues. It is distinguished from the Myocardial Tissue.