Tube Growth of Solanum Chacoense
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollen Tube Guidance by Pistils Ensures Successful Double Fertilization Ravishankar Palanivelu∗ and Tatsuya Tsukamoto
Advanced Review Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization Ravishankar Palanivelu∗ and Tatsuya Tsukamoto Sexual reproduction in flowering plants is unique in multiple ways. Distinct multicellular gametophytes contain either a pair of immotile, haploid male gametes (sperm cells) or a pair of female gametes (haploid egg cell and homodiploid central cell). After pollination, the pollen tube, a cellular extension of the male gametophyte, transports both male gametes at its growing tip and delivers them to the female gametes to affect double fertilization. The pollen tube travels a long path and sustains its growth over a considerable amount of time in the female reproductive organ (pistil) before it reaches the ovule, which houses the female gametophyte. The pistil facilitates the pollen tube’s journey by providing multiple, stage-specific, nutritional, and guidance cues along its path. The pollen tube interacts with seven different pistil cell types prior to completing its journey. Consequently, the pollen tube has a dynamic gene expression program allowing it to continuously reset and be receptive to multiple pistil signals as it migrates through the pistil. Here, we review the studies, including several significant recent advances, that led to a better understanding of the multitude of cues generated by the pistil tissues to assist the pollen tube in delivering the sperm cells to the female gametophyte. We also highlight the outstanding questions, draw attention to opportunities created by recent advances and point to approaches that could be undertaken to unravel the molecular mechanisms underlying pollen tube–pistil interactions. 2011 Wiley Periodicals, Inc. -

Ap09 Biology Form B Q2
AP® BIOLOGY 2009 SCORING GUIDELINES (Form B) Question 2 Discuss the patterns of sexual reproduction in plants. Compare and contrast reproduction in nonvascular plants with that in flowering plants. Include the following topics in your discussion: (a) alternation of generations (b) mechanisms that bring female and male gametes together (c) mechanisms that disperse offspring to new locations Four points per part. Student must write about all three parts for full credit. Within each part it is possible to get points for comparing and contrasting. Also, specific points are available from details provided about nonvascular and flowering plants. Discuss the patterns of sexual reproduction in plants (4 points maximum): (a) Alternation of generations (4 points maximum): Topic Description (1 point each) Alternating generations Haploid stage and diploid stage. Gametophyte Haploid-producing gametes. Dominant in nonvascular plants. Double fertilization in flowering plants. Gametangia; archegonia and antheridia in nonvascular plants. Sporophyte Diploid-producing spores. Heterosporous in flowering plants. Flowering plants produce seeds; nonvascular plants do not. Flowering plants produce flower structures. Sporangia (megasporangia and microsporangia). Dominant in flowering plants. (b) Mechanisms that bring female and male gametes together (4 points maximum): Nonvascular Plants (1 point each) Flowering Plants (1 point each) Aquatic—requires water for motile sperm Terrestrial—pollination by wind, water, or animal Micropyle in ovule for pollen tube to enter Pollen tube to carry sperm nuclei Self- or cross-pollination Antheridia produce sperm Gametophytes; no antheridia or archegonia Archegonia produce egg Ovules produce female gametophytes/gametes Pollen: male gametophyte that produces gametes © 2009 The College Board. All rights reserved. Visit the College Board on the Web: www.collegeboard.com. -

Lesson 6: Plant Reproduction
LESSON 6: PLANT REPRODUCTION LEVEL ONE Like every living thing on earth, plants need to make more of themselves. Biological structures wear out over time and need to be replaced with new ones. We’ve already looked at how non-vascular plants reproduce (mosses and liverworts) so now it’s time to look at vascular plants. If you look back at the chart on page 17, you will see that vascular plants are divided into two main categories: plants that produce seeds and plants that don’t produce seeds. The vascular plants that do not make seeds are basically the ferns. There are a few other smaller categories such as “horse tails” and club mosses, but if you just remember the ferns, that’s fine. So let’s take a look at how ferns make more ferns. The leaves of ferns are called fronds, and brand new leaves that have not yet totally uncoiled are called fiddleheads because they look like the scroll-shaped end of a violin. Technically, the entire frond is a leaf. What looks like a stem is actually the fern’s equivalent of a petiole. (Botanists call it a stipe.) The stem of a fern plant runs under the ground and is called a rhizome. Ferns also have roots, like all other vascular plants. The roots grow out from the bottom of the rhizome. Ferns produce spores, just like mosses do. At certain times of the year, the backside of some fern fronds will be covered with little dots called sori. Sori is the plural form, meaning more than one of them. -

Two Expansin Genes, Atexpa4 and Atexpb5, Are Redundantly Required for Pollen Tube Growth and Atexpa4 Is Involved in Primary Root Elongation in Arabidopsis Thaliana
G C A T T A C G G C A T genes Article Two Expansin Genes, AtEXPA4 and AtEXPB5, Are Redundantly Required for Pollen Tube Growth and AtEXPA4 Is Involved in Primary Root Elongation in Arabidopsis thaliana Weimiao Liu 1,2, Liai Xu 1,2 , Hui Lin 3 and Jiashu Cao 1,2,4,* 1 Laboratory of Cell and Molecular Biology, Institute of Vegetable Science, Zhejiang University, Hangzhou 310058, China; [email protected] (W.L.); [email protected] (L.X.) 2 Key Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Ministry of Agriculture, Hangzhou 310058, China 3 Crop Research Institute, Fujian Academy of Agricultural Sciences, Fuzhou 350013, China; [email protected] 4 Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Hangzhou 310058, China * Correspondence: [email protected]; Tel.: +86-131-8501-1958 Abstract: The growth of plant cells is inseparable from relaxation and expansion of cell walls. Expansins are a class of cell wall binding proteins, which play important roles in the relaxation of cell walls. Although there are many members in expansin gene family, the functions of most expansin genes in plant growth and development are still poorly understood. In this study, the functions of two expansin genes, AtEXPA4 and AtEXPB5 were characterized in Arabidopsis thaliana. AtEXPA4 and AtEXPB5 displayed consistent expression patterns in mature pollen grains and pollen tubes, but AtEXPA4 also showed a high expression level in primary roots. Two single mutants, atexpa4 and atexpb5, showed normal reproductive development, whereas atexpa4 atexpb5 double mutant was defective in pollen tube growth. Moreover, AtEXPA4 overexpression enhanced primary root Citation: Liu, W.; Xu, L.; Lin, H.; Cao, elongation, on the contrary, knocking out AtEXPA4 made the growth of primary root slower. -

Physiological Importance of Pectin Modifying Genes During Rice Pollen Development
International Journal of Molecular Sciences Article Physiological Importance of Pectin Modifying Genes During Rice Pollen Development 1, 2, 1, 1 1 Yu-Jin Kim y , Ho Young Jeong y, Seung-Yeon Kang y, Jeniffer Silva , Eui-Jung Kim , Soon Ki Park 3, Ki-Hong Jung 1,* and Chanhui Lee 2,* 1 Graduate School of Biotechnology & Crop Biotech Institute, Kyung Hee University, Yongin 17104, Korea; [email protected] (Y.-J.K.); [email protected] (S.-Y.K.); jeniff[email protected] (J.S.); [email protected] (E.-J.K.) 2 Department of Plant & Environmental New Resources, College of Life Sciences, Kyung Hee University, Yongin 17104, Korea; [email protected] 3 School of Applied Biosciences, Kyungpook National University, Daegu 41566, Korea; [email protected] * Correspondence: [email protected] (K.-H.J.); [email protected] (C.L.); Tel.:+82-31-201-3474 (K.-H.J.) These authors contributed equally to this work. y Received: 23 May 2020; Accepted: 7 July 2020; Published: 8 July 2020 Abstract: Although cell wall dynamics, particularly modification of homogalacturonan (HGA, a major component of pectin) during pollen tube growth, have been extensively studied in dicot plants, little is known about how modification of the pollen tube cell wall regulates growth in monocot plants. In this study, we assessed the role of HGA modification during elongation of the rice pollen tube by adding a pectin methylesterase (PME) enzyme or a PME-inhibiting catechin extract (Polyphenon 60) to in vitro germination medium. Both treatments led to a severe decrease in the pollen germination rate and elongation. -

How Flowering Plants Discriminate Between Self and Non-Self Pollen to Prevent Inbreeding TEH-HUI KAO and ANDREW G
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 12059-12065, October 1996 Symposium Paper This paper was presented at a symposium entitled "Frontiers in Plant Biology: How Plants Communicate, " organized by Hans Kende and held at the 133rd Annual Meeting of the National Academy of Sciences on April 30, 1996. How flowering plants discriminate between self and non-self pollen to prevent inbreeding TEH-HUI KAO AND ANDREW G. MCCUBBIN Department of Biochemistry and Molecular Biology, 403 Althouse Laboratory, Pennsylvania State University, University Park, PA 16802 ABSTRACT Flowering plants have evolved various ge- heteromorphic types. In the homomorphic type, flowers of the netic mechanisms to circumvent the tendency for self- same species have the same morphological type, whereas in the fertilization created by the close proximity of male and female heteromorphic type, flowers of the same species can have two reproductive organs in a bisexual flower. One such mecha- or three different morphological types, and pollination is nism is gametophytic self-incompatibility, which allows the compatible only between flowers of different morphological female reproductive organ, the pistil, to distinguish between types (2). The homomorphic type is further classified into self pollen and non-self pollen; self pollen is rejected, whereas gametophytic and sporophytic types based on whether the non-self pollen is accepted for fertilization. The Solanaceae pollen behavior in self-incompatibility interactions is deter- family has been used as a model to study the molecular and mined by the genotype of the pollen itself (gametophytic) or biochemical basis of self/non-self-recognition and self- by the genotype of the plant from which the pollen is derived rejection. -

Chapter 12: Life Cycles: Meiosis and the Alternation of Generations
Chapter 12 Life Cycles: Meiosis and the Alternation of Generations LIFE CYCLES TRANSFER GENETIC INFORMATION Asexual Reproduction Transfers Unchanged Genetic Information through Mitosis Sexual Reproduction Produces New Information through Meiosis and Fertilization ALTERNATION BETWEEN DIPLOID AND HAPLOID GENERATIONS Plants Vary in the Details of Their Life Cycles Sexual Cycles Can Be Heterosporic or Homosporic Only One Generation Is Multicellular in Zygotic or Gametic Life Cycles The Diploid Generation Has Become Dominant over Evolutionary Time SUMMARY 1 KEY CONCEPTS 1. Life perpetuates itself through reproduction, which is the transfer of genetic information from one generation to the next. This transfer is our definition of life cycle. Reproduction can be asexual or sexual. 2. Asexual reproduction requires a cell division know as mitosis. Asexual reproduction offers many advantages over sexual reproduction, one of which is that it requires only a single parent. A significant disadvantage of asexual reproduction is the loss of genetic diversity and the likelihood of extinction when the environment changes. 3. Sexual reproduction involves the union of two cells, called gametes, which are usually produced by two different individuals. Another kind of cell division, known as meiosis, ultimately is necessary to produce gametes. 4. Every species in the kingdom Plantae has both diploid and haploid phases--that is, plants whose cells are all diploid or all haploid. These phases are called generations, and they alternate with each other over time. 5. The fossil record reveals that the most recent groups to evolve have sporic life cycles, in which the gametophyte (haploid) generation is relatively small and the sporophyte (diploid) generation is dominant in terms of size, complexity, and longevity. -
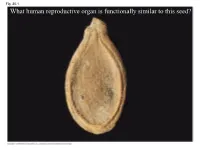
What Human Reproductive Organ Is Functionally Similar to This Seed?
Fig. 30-1 What human reproductive organ is functionally similar to this seed? Overview: Transforming the World • Seeds changed the course of plant evolution, enabling their bearers to become the dominant producers in most terrestrial ecosystems • A seed consists of an embryo and nutrients surrounded by a protective coat Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-1 What human reproductive organ is functionally similar to this seed? Seeds and pollen grains are key adaptations for life on land • In addition to seeds, the following are common to all seed plants – Reduced gametophytes – Heterospory – Ovules – Pollen Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Advantages of Reduced Gametophytes • The gametophytes of seed plants develop within the walls of spores that are retained within tissues of the parent sporophyte • Protect female gametophyte from UV and desiccation (good for the land life!) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-2 PLANT GROUP Mosses and other Ferns and other seedless Seed plants (gymnosperms and angiosperms) nonvascular plants vascular plants Reduced, independent Reduced (usually microscopic), dependent on surrounding (photosynthetic and Gametophyte Dominant sporophyte tissue for nutrition free-living) Sporophyte Reduced, dependent on Dominant Dominant gametophyte for nutrition Gymnosperm Angiosperm Sporophyte Microscopic female (2n) gametophytes (n) inside ovulate cone Microscopic Sporophyte -

Plant Reproduction Asexual Reproduction
Plant Reproduction Asexual Reproduction • Asexual reproduction is natural “cloning.” Parts of the plant, such as leaves or stems, produce roots and become an independent plant. Sexual Reproduction • Sexual reproduction requires fusion of male cells in the pollen grain with female cells in the ovule. Terms to know: • Haploid: having a single set of chromosomes in each cell. • Diploid: having two sets of chromosomes in each cell, one set from each parent. • Mitosis: cell division, which produces two genetically identical diploid cells. • Meiosis: reduction division, which produces four haploid reproductive cells. Terms to know: • Spore: haploid reproductive cell that leads to a gametophyte in plant alternation of generations. • Gamete: mature haploid male or female germ cell able to unite with another of the opposite sex in sexual reproduction to form a zygote. • Zygote: diploid, eukaryotic cell formed during fertilization event between two gametes, combining DNA of each gamete, containing the genetic information to form a new individual. Terms to know: • Sporophyte: diploid, multicellular stage which develops from zygote, produced when a haploid female cell is fertilized by a haploid male cell, produces haploid spores by meiosis. • Gametophyte: haploid, multicellular stage, develops from a spore by mitosis, produces haploid gametes by mitosis. Plant Life Cycle Animals vs. Plants Plant Reproduction Animal Reproduction Alternation of No alternation of Life cycle generations generations Gametes Haploid gametes Haploid gametes Spores Haploid spores N/A (no spores) Gametes made Haploid gametophyte, Diploid organism, by by by mitosis meiosis Diploid sporophyte, by Spores made by N/A (no spores) meiosis Alternation of Generations • Plants have a double life cycle with two forms: • Sporophyte • Gametophyte Non-flowering plants • Mosses, ferns, and related plants have motile, swimming sperm. -

Effects of Pollen Size on Siring Success in the Hermaphroditic Common
RESEARCH ARTICLE AMERICAN JOURNAL OF BOTANY INVITED PAPER For the Special Issue: Ecology and Evolution of Pollen Performance Pollen competition in style: Eff ects of pollen size on siring success in the hermaphroditic common morning glory, Ipomoea purpurea 1 Britnie McCallum and Shu-Mei Chang 2 PREMISE OF THE STUDY: Pollen size varies greatly among fl owering plant species and has been shown to infl uence the delivery of sperm cells to the eggs. Relatively little is known, however, about the functional signifi cance of within-species genetic variation in pollen size. This study tests whether pollen size infl uences the relative siring success of a pollen donor during in vivo pollen competition experiments. METHODS: We used two groups of Ipomoea purpurea plants genetically divergent in their pollen sizes and applied equal number of pollen grains from one large-pollen and one small-pollen donor onto the same stigma. Using microsatellite genetic markers, we identifi ed the pollen parent of each of the result- ing progeny to determine the relative siring success of the competing donors. Competitions between donors of equal-sized pollen served as a control. KEY RESULTS: Diff erences in pollen size signifi cantly aff ected the relative siring success of a pollen donor; larger-grained individuals outcompeted smaller- grained competitors but not equal-sized competitors. Relative siring success, however, sometimes varied across diff erent pollen recipients. CONCLUSIONS: Pollen size can infl uence the relative siring success of diff erent individuals competing on the same stigma during postpollination processes. However, other factors, such as pollen–pistil interaction and environmental conditions, are likely to infl uence these competitions as well. -

Pollen Tube Development in Petunia Hybrida Following Compatible and Incompatible Intraspecific Matings
J. Cell Sd. 47, 365-383 (1981) 365 Printed in Great Britain © Company of Biologists Limited ig8i POLLEN TUBE DEVELOPMENT IN PETUNIA HYBRIDA FOLLOWING COMPATIBLE AND INCOMPATIBLE INTRASPECIFIC MATINGS MARIA HERRERO* AND H. G. DICKINSON Department of Botany, Plant Science Laboratories, University of Reading, Whiteknights, Reading RG6 2AS, U.K. SUMMARY Pollen tubes formed following compatible and incompatible intraspecific matings in Petunia have been examined with light and electron microscopes. Compatible and incom- patible tubes develop in an identical fashion on the stigma but, on entry into the top 1 mm of the stylar transmitting tissue changes occur both to the cytology of the tubes and their rates of growth. The early cytological changes are common to tubes of both compatibilities but, although both types of tube accelerate on entry into the style, incompatible tubes grow more slowly than compatible. Cytological differences became apparent between compatible and incompatible tubes following a short period of growth in the style, the latter possessing thicker cell walls and a cytoplasm packed with both organelles and reserves. Incompatible tubes subsequently burst or simply cease growth and die. The characteristic image afforded by this cytoplasm resembles that of burst or dead compatible tubes, except in that proportions of the cell com- ponents may differ. These data are discussed in terms of current models proposed to explain pollen tube growth and the operation of the self-incompatibility response in Petunia. INTRODUCTION As in many plants with gametophytic control of pollen compatibility with respect to the style, pollen tubes of Petunia formed following a self-mating fail to reach the ovary. -

Ions and Pollen Tube Growth
Plant Cell Monogr (3) R. Malhó: The Pollen Tube DOI 10.1007/7089_043/Published online: 26 January 2006 © Springer-Verlag Berlin Heidelberg 2006 Ions and Pollen Tube Growth Peter K. Hepler1 (u) · Alenka Lovy-Wheeler1 · Sylvester T. McKenna2 · Joseph G. Kunkel1 1Department of Biology, University of Massachusetts, Amherst, MA 01003, USA [email protected] 2Department of Biology, Long Island University, Brooklyn, NY 11201, USA Abstract Ions play a crucial role in the control of pollen tube growth. In this review we focus on four that seem especially important: calcium (Ca2+), protons (H+), potassium (K+), and chloride (Cl–). Ca2+ in the extracellular medium is essential for growth; it forms a steep intracellular tip-focused gradient, and exhibits a prominent extracellular tip-directed Ca2+ influx. pH is also essential for growth. H+ form an intracellular gra- dient consisting of a slightly acidic domain at the extreme apex and an alkaline band located along the clear zone. H+ also exhibit an apical influx, but in contrast to Ca2+ show an efflux along the clear zone, in the region occupied by the intracellular alka- line band. K+ and anions (possibly Cl–)appeartoparticipateinthegrowthprocess, as evidenced by the striking extracellular fluxes that are associated with tube elonga- tion. K+ exhibits an apical influx, while an anion displays an apical efflux. An exciting finding has been the discovery that pollen tube growth oscillates in rate, as do all the ionic expressions noted above. While the ionic activities and fluxes show the same period as growth, they usually do not show the same phase. The exploration of phase rela- tionships, using cross-correlation analysis, reveals that most ion expressions lag growth.