Biological Warfare Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Francisella Tularensis Blue-Grey Phase Variation Involves Structural
Francisella tularensis blue-grey phase variation involves structural modifications of lipopolysaccharide O-antigen, core and lipid A and affects intramacrophage survival and vaccine efficacy THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Shilpa Soni Graduate Program in Microbiology The Ohio State University 2010 Master's Examination Committee: John Gunn, Ph.D. Advisor Mark Wewers, M.D. Robert Munson, Ph.D. Copyright by Shilpa Soni 2010 Abstract Francisella tularensis is a CDC Category A biological agent and a potential bioterrorist threat. There is no licensed vaccine against tularemia in the United States. A long- standing issue with potential Francisella vaccines is strain phase variation to a grey form that lacks protective capability in animal models. Comparisons of the parental strain (LVS) and a grey variant (LVSG) have identified lipopolysaccharide (LPS) alterations as a primary change. The LPS of the F. tularensis variant strain gains reactivity to F. novicida anti-LPS antibodies, suggesting structural alterations to the O-antigen. However, biochemical and structural analysis of the F. tularensis LVSG and LVS LPS demonstrated that LVSG has less O-antigen but no major O-antigen structural alterations. Additionally, LVSG possesses structural differences in both the core and lipid A regions, the latter being decreased galactosamine modification. Recent work has identified two genes important in adding galactosamine (flmF2 and flmK) to the lipid A. Quantitative real-time PCR showed reduced transcripts of both of these genes in the grey variant when compared to LVS. Loss of flmF2 or flmK caused less frequent phase conversion but did not alter intramacrophage survival or colony morphology. -

The History of Chemical and Biological Warfare: an American
History of Chemical and Biological Warfare: An American Perspective Chapter 2 HISTORY OF CHEMICAL AND BIOLOGICAL WARFARE: AN AMERICAN PERSPECTIVE JEFFERY K. SMART, M.A.* INTRODUCTION PRE–WORLD WAR I DEVELOPMENTS WORLD WAR I THE 1920S: THE LEAN YEARS THE 1930S: THE GROWING THREAT OF CHEMICAL AND BIOLOGICAL WARFARE THE 1940S: WORLD WAR II AND THE NUCLEAR AGE THE 1950S: HEYDAY OF THE CHEMICAL CORPS THE 1960S: DECADE OF TURMOIL THE 1970S: THE NEAR END OF THE CHEMICAL CORPS THE 1980S: THE RETURN OF THE CHEMICAL CORPS THE 1990S: THE THREAT MATERIALIZES SUMMARY *Command Historian, U.S. Army Chemical and Biological Defense Command, Aberdeen Proving Ground, Maryland 21010-5423 9 Medical Aspects of Chemical and Biological Warfare INTRODUCTION Webster’s Ninth New Collegiate Dictionary defines or biological warfare went virtually unnoticed by the term “chemical warfare,” first used in 1917, the U.S. Army. By the end of World War I, the situ- as “tactical warfare using incendiary mixtures, ation had drastically changed. Chemical warfare smokes, or irritant, burning, poisonous, or asphyx- had been used against and by American soldiers iating gases.” A working definition of a chem- on the battlefield. Biological warfare had been used ical agent is “a chemical which is intended for covertly on several fronts. In an effort to determine use in military operations to kill, seriously injure, what had gone wrong with their planning and train- or incapacitate man because of its physiological ing, U.S. Army officers prepared a history of chemi- effects. Excluded from consideration are riot con- cal and biological warfare. To their surprise, they trol agents, chemical herbicides and smoke found numerous documented cases of chemical and and flame materials.”1(p1-1) Chemical agents were biological agents having been used or proposed to usually divided into five categories: nerve agents, influence the outcome of a battle or campaign. -

Rudderless: the Chemical Weapons Convention at 1 ½
Rudderless: The Chemical Weapons Convention At 1 ½ Amy E. Smithson Report No. 25 September 1998 Copyright© 1998 11 Dupont Circle, NW Ninth Floor Washington, DC 20036 phone 202.223.5956 fax 202.238.9604 http://www.stimson.org email [email protected] Rudderless: The Chemical Weapons Convention At 1 1/2 Amy E. Smithson INTRODUCTION On the 29th of April 1997, the majority of the world’s nations joined to activate an arms control and nonproliferation accord that will gradually compel the elimination of one of the most abhorred classes of weapons of all times. Previously, the international community had fallen short of the mark in efforts to try to abolish poison gas, despite the opprobrium following its widespread use in World War I.1 The new Chemical Weapons Convention (CWC) extends the no use-prohibitions of the 1925 Geneva Protocol2 to outlaw the development, acquisition, production, transfer, and stockpiling of chemical weapons as well. The CWC requires the destruction of chemical weapons production facilities and arsenals over a ten-year period, and countries will witness the shrinking numbers of poison gas factories and munitions. A less tangible function of the CWC, but one that may turn out to be equally valued over the long term is that the CWC will help redefine how states assure their national security. The CWC requires nations to declare activities that were previously considered state secrets and private business information. The treaty authorizes routine and challenge inspections to monitor compliance with its prohibitions. Instead of building large caches of arms, the CWC’s verification processes give governments reason to be confident that managed transparency—a limited waiver of state sovereignty—can enhance national and international security. -

Biological Weapons During the Cold War
Biological Weapons During the Cold War Lecture No. 4 1. Outline • At the end of World War II – Slides 2 - 3 • The US Programme – Slides 4 - 11 • The Anti-Crop Aspect of US Activities – Slides 11 - 16 • The Soviet Programme – Slides 17 - 20 2. At the End of the War • Canadian concerns – “…in the spring of 1944…intelligence reports indicated that the German military had added botulinus toxin to its arsenal…” – “…Canada’s military leaders were also impressed by …experiments which had demonstrated why botulinus toxin was an effective weapon…” 3. Botulinum Toxin as a Biological Weapon • “Botulinum toxin is the most poisonous substance known….the toxin is a zinc proteinase that cleaves 1 or more of the fusion proteins by which neuronal vesicles release acetylcholine into the neuromuscular junction.” • “…In a large outbreak of botulism, the need for mechanical ventilators, critical care beds, and skilled personnel might quickly exceed local capacity and persist for weeks or months…” 4. Stages of the US Programme • Research and Planning (1946 – 49) • Expansion during the Korean War (1950 – 53) • Reorganisation (1954 – 58) • The Limited War Period (1959 – 62) • Adaptation to Counter insurgency (1963 – 68) • Disarmament and Phased Down (1973 – 77) 5. 1946 - 1949 • “When World War II ended, the CWS (Chemical Warfare Service) had as its major mission preparedness for CW and BW in the context of a policy of retaliation only…” • …Activities were concentrated on BW agent research and defensive aspects; some applied research on dissemination devices; the collation and digestion of the large scale R&D effort carried out during World War II; and the formation of sound research and development program frameworks...” 6. -

97 STAT. 757 Public Law 98-115 98Th Congress an Act
PUBLIC LAW 98-115—OCT. 11, 1983 97 STAT. 757 Public Law 98-115 98th Congress An Act To authorize certain construction at military installations for fiscal year 1984, and for Oct. 11, 1983 other purposes. [H.R. 2972] Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled. That this Act may Military be cited as the "Military Construction Authorization Act, 1984'\ Au'thorizSn Act, 1984. TITLE I—ARMY AUTHORIZED ARMY CONSTRUCTION AND LAND ACQUISITION PROJECTS SEC. 101. The Secretary of the Army may acquire real property and may carry out military construction projects in the amounts shown for each of the following installations and locations: INSIDE THE UNITED STATES UNITED STATES ARMY FORCES COMMAND Fort Bragg, North Carolina, $31,100,000. Fort Campbell, Kentucky, $15,300,000. Fort Carson, Colorado, $17,760,000. Fort Devens, Massachusetts, $3,000,000. Fort Douglas, Utah, $910,000. Fort Drum, New York, $1,500,000. Fort Hood, Texas, $76,050,000. Fort Hunter Liggett, California, $1,000,000. Fort Irwin, California, $34,850,000. Fort Lewis, Washington, $35,310,000. Fort Meade, Maryland, $5,150,000. Fort Ord, California, $6,150,000. Fort Polk, Louisiana, $16,180,000. Fort Richardson, Alaska, $940,000. Fort Riley, Kansas, $76,600,000. Fort Stewart, Georgia, $29,720,000. Presidio of Monterey, California, $1,300,000. UNITED STATES ARMY WESTERN COMMAND Schofield Barracks, Hawaii, $31,900,000. UNITED STATES ARMY TRAINING AND DOCTRINE COMMAND Carlisle Barracks, Pennsylvania, $1,500,000. Fort Benjamin Harrison, Indiana, $5,900,000. -

Disposal of Chemical Weapons: Alternative Technologies (Part 4 of 8)
Chapter 2 The Army’s Chemical Weapons Disposal Program Percentage THE U.S. ARMY’S CHEMICAL Site Iocation of total WEAPONS STOCKPILE Tooele Army Depot, UT,. 42.3 Pine Bluff Arsenal, AR. 12.0 Umatilla Depot, OR... 11.6 Geography and Distribution Pueblo Depot, CO. 9.9 Anniston Army Depot, AL.. 7.1 The chemical weapons (CW) stockpile is located Johnston Island, South Pacific . 6.6 on Army bases at eight continental U.S. sites (see Aberdeen Proving Ground, MD. 5.0 Newport Army Ammunition Plant, IN...... 3.9 figure 2-1) and at Johnston Island in the Pacific Lexington-Blue Grass Army Depot, KY. 1.6 Ocean (717 nautical miles southwest of Hawaii). It is distributed as follows (by percentage of chemical The stockpile includes chemical agents stored in agent): bulk containers without explosives and propellants, as well as rockets, land mines, mortars, cartridges, Figure 2-1—U.S. Chemical Weapons Stockpile Distribution I Newport Army I Ammunition Plant I VX - TC / (3.9%) Umatilla Depot HD - TC GB -P, R, B VX - P, R, M, ST (1 1.6%) Tooele Army Depot Lexington- H-P Blue Grass Army HD -C, P, TC HT -C, P GB -C, P, R, B, TC GB - P, R, TC (42.3%) VX -P, R (1 .6%) Pueblo Depot HD -C, P Anniston Army HT - C Depot (9.9%) Pine Bluff HD -C, P, TC Arsenal HT - C HD -C, TC GB -C, P, R GB, VX, H, HD, HT = Chemical agents. HT - TC VX -P, R, M GB - R (7.1%) TC = Ton container VX - R, M R = Rockets (12.0%) M = Mines ST= Spray tanks B = Bombs C = Cartridges P = Projectiles SOURCE: U.S. -
![82 Stat. ] Public Law 90-408-July 21, 1968 367](https://docslib.b-cdn.net/cover/3002/82-stat-public-law-90-408-july-21-1968-367-4083002.webp)
82 Stat. ] Public Law 90-408-July 21, 1968 367
82 STAT. ] PUBLIC LAW 90-408-JULY 21, 1968 367 (2) Section 5814 of such title is amended by striking out paragraph 5°us*c's3^i^ 40, and by inserting in lieu thereof the following new paragraph: "'(40) Deputy Director, National Science Foundation." (3) Section 5316 of such title is amended by striking out paragraph ^° ^*^* '*^'^' (66), and by inserting in lieu thereof the following new paragraph: "(66) Assistant Directors, National Science Foundation (4)." (4) The amendments made by this subsection (and the amendments Effective date. made by sections 3 and 4 of this Act insofar as they relate to rates of basic pay) shall take effect on the first day of the first calendar month which begins on or after the date of the enactment of this Act. (b) Section 902(c) of the National Defense Education Act of 1958 ^ ^'^^ l^^^- is amended by striking out "$50" and inserting in lieu thereof "$100". SEC. 16. Except as otherwise specifically provided therein, the amendments made by this Act are intended to continue in effect under the National Science Foundation Act of 1950 the existing offices, pro cedures, and organization of the National Science Foundation as provided by such Act, part II of Reorganization Plan Numbered 2 of 1962, and Reorganization Plan Numbered 5 of 1965. From and after ^^ ^t^' 1254. the date of the enactment of this Act, part II of Reorganization Plan note. ^^^'^ Numbered 2 of 1962, and Reorganization Plan Numbered 5 of 1965, 79 stat. 1323. shall be of no force or effect; but nothing in this Act shall alter or ^^ "^^ ^^^^ affect any transfers of functions made by part I of such Reorganiza note. -
Textile Visit *Pine Bluff Arsenal Days Without a Record- CSM Able Injury Are 26
Ju Junene 8, 2017 Vol umeume 33,33, NumberNumber 66 PBA PBA CYS gets top marks during inspection SAFETY By Rachel Selby ily Morale, Welfare and lent on-post support,” she she said. “He was very in Pine Bluff Arsenal Recreation. said. “This was a wonder- depth. We gave him GLANCE Child, Youth and School Brodnax said a lot of ful thing we were able to binder after binder full of Services completed an the findings were dupli- do.” information for him to re- unannounced inspection cate findings from differ- Johnson said this an- view. He went through conducted by U.S. Army ent program areas. “Most nual inspection looks at everything.” Installation Management findings were tied to an- everything. “This inspec- Since training was one Command. nual training that we tion covers CYSS, Child of the findings, each di- The program received knew had lapsed by two Development Center, rector – CDC, School Age Safety Element for top marks for the inspec- weeks because we chose School Age programs and and Youth – will have tion, which was held May to consolidate all of the Youth programs,” she training as one of their fiscal year 2017: 8-10. This is one of four program training,” she said. “The inspector duties. Lost Day Case: annual inspections that said. “Other findings were looked at everything – “We are changing 0.38 are done to obtain De- corrected on the spot employee files for train- things up. This is some- partment of Defense cer- and/or will be corrected ing, background checks, thing we had to do and Award Goal: 0.61 tification to operate. -
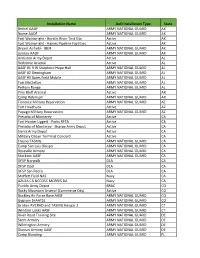
Additional Army Installations Being Assessed for PFAS Release.Xlsx
Installation Name DoD Installation Type State Bethel AAOF ARMY NATIONAL GUARD AK Nome AAOF ARMY NATIONAL GUARD AK Fort Wainwright - Gerstle River Test Site Active AK Fort Wainwright - Haines Pipeline Facilities Active AK Bryant Airfield- JBER ARMY NATIONAL GUARD AK Juneau AAOF ARMY NATIONAL GUARD AK Anniston Army Depot Active AL Redstone Arsenal Active AL AASF #1 R W Shepherd Hope Hull ARMY NATIONAL GUARD AL AASF #2 Birmingham ARMY NATIONAL GUARD AL AASF #3 Bates Field Mobile ARMY NATIONAL GUARD AL Fort McClellan ARMY NATIONAL GUARD AL Pelham Range ARMY NATIONAL GUARD AL Pine Bluff Arsenal Active AR Camp Robinson ARMY NATIONAL GUARD AR Florence Military Reservation ARMY NATIONAL GUARD AZ Fort Huachuca Active AZ Papago Military Reservation ARMY NATIONAL GUARD AZ Presidio of Monterey Active CA Fort Hunter Liggett - Parks RFTA Active CA Presidio of Monterey - Sharpe Army Depot Active CA Sierra Army Depot Active CA Military Ocean Terminal Concord Active CA Fresno TASMG ARMY NATIONAL GUARD CA Camp San Luis Obispo ARMY NATIONAL GUARD CA Roseville Armory ARMY NATIONAL GUARD CA Stockton AASF ARMY NATIONAL GUARD CA DFSP Norwalk DLA CA DFSP Ozol DLA CA DFSP San Pedro DLA CA Moffett Field NAS Navy CA AZUSA CA NCCOSC MORRIS DA Navy CA Pueblo Army Depot BRAC CO Rocky Mountain Arsenal (Commerce City) Active CO Buckley Air Force Base AASF ARMY NATIONAL GUARD CO Gypsum (HAATS) ARMY NATIONAL GUARD CO Groton AVCRAD and TASMG Hangar 2 ARMY NATIONAL GUARD CT Windsor Locks AASF ARMY NATIONAL GUARD CT River Road Training Site ARMY NATIONAL GUARD DE Stern Armory -
Weapons of Mass Destruction Programs and the Legacy They Left Behind
This document contains parts of the introductory essay on this collection. The full version can be found on: https://primarysources.brillonline.com/browse/weapons-of-mass-destruction (background tab). America's Weapons of Mass Destruction Programs And The Legacy They Left Behind Matthew M. Aid Table of contents •Introduction ◦The U.S. Government's Fixation with WMD Secrecy ◦Key Findings Contained in this Collection ◦Tactical Nuclear Weapons in Europe and Asia ◦Security and Safety Threats to U.S. Nuclear Weapons •Keeping U.S. Chemical and Biological Weapons Programs Secret ◦The Security and Safety of the U.S. Chemical Weapons Stockpile •The U.S. Biological Warfare Program •The Toxic Legacy of America's WMD Programs Introduction This document collection has been compiled for the simple purpose of helping researchers students bypass the vast amount of secrecy surrounding the subject of how the U.S. built up the world's largest arsenal of nuclear, chemical and biological weapons during the Cold War. It is worth remembering that at its peak in 1967, the U.S. nuclear arsenal consisted of 31,255 nuclear weapons with an aggregate destructive power of 12,786 megatons, which was more than sufficient to wipe out all of humanity several hundred times over. But that was not all. Also hidden away in earth-covered storage bunkers spread throughout the U.S. as well as Germany and Okinawa were over 40,000 tons of chemical weapons, as well as thousands of specially designed bombs that could be filled in short order with even deadlier biological warfare agents, such as weaponized versions of the anthrax virus and tularense (rabbit fever bacteria). -
Drop Kick,M 1956
v RCC1.950228.006 UNCLASSTFIED f SUMMARY OF MAJOR. EVENTS and PROBLEMS United States Army Chemical Corps (U) Fiscal Year 1959 January 1960 260003 w sew U. S. Army Chemical Corps Historical Office Army Chemical Center, Maryland SWYOF MAJm EVENTS AND AIOBLW (Reports Control Syrnbol CSHIS-6) UNITED STATES ARMY CHEMICAL CORPS Fiscal Year 1959 Reproduction of this document in whole or in part is prohibited except with permission of the issuing office [I. So Army Chemical Corps Historical Office January 1960 PAGE 1 OF 1*1 - PAGES r COPY 1 OF 35 COPIES -uimm UNCLASSIFIED Page 111. RESEARCH, DEVELOPMENT AND ENGINEERING. ............ 88 A. Administration ...................... 88 1. Thirteenth Tripartite Conference ........... 95 B. Technical Operations ................... 96 1. cs...............,.......... 96 2. Tularemia. ...................... 98 3. Yellow Fever ..................... 10 1 4. AnticropBW. ..................... 105 5. BW Spray Tests .................... 107 6. Mask, Protective, Field, M17 ............. 109 7. Gas Mask Vulcanizer, . e . , 110 8. Automatic G-Agent Alarm, e e e e . 111 9. GBSomb........~...”~........~. 112 10. PN Incendiary Mixture e e . 114 11. Burster, Incendiary, Field (M4). e . e . 116 <.::,!b, :. 12. Protection and Treatment Set M5A2. e . I . 117 -. 13. Truck-Mounted Decontaminating Apparatus. 118 14. Decontaminating Slurry Antiset e e e e a . 119 15. White Phosphorus Grenade e . * . - 120 16. Shipping Guard for the 1,000-lb. Bomb Cluster. 121 17. Carbon Monoxide Detector Kit * . e . 122 IV. MATmIEL- - a e e LI e * * e *. 123 A. Managbment and Organization. e . e . 123 1. Pr3curement and Production Capability. ........ 123 2. Organization and Management. ............. 133 B. Procurement and Production e . 144 1. Operation BLACK MAGIC. -
Cschemical Stockpile Emerg Eency Prpeparednesps Program
Prepared for the Chemical Stockpile Emergency Preparedness Program by Argonne National Laboratory Risk Communication and Management CSCHEMICAL STOCKPILE EMERGEENCY PRPEPAREDNESPS PROGRAM A Brief History of Chemical Weapons Chemical weapons have been around for a long time. As far back as 10,000 B.C., Stone Age hunters used poison-tipped arrows against game animals to help feed themselves. The fi rst recorded instance of gas warfare was in the fi fth century B.C. during the Peloponnesian War, when a combination of pitch and sulfur smoke was used. Other confl icts during succeeding centuries saw the use of smoke and fl ame. A renewed interest in chemicals as military weapons occurred with the birth of modern inorganic chemistry during the late 18th and early 19th centuries and the growth of organic chemistry in Germany during the late 19th and early 20th centuries. World War I began amid a continuing debate about the morality of chemical warfare. In April 1915, German units released chlorine gas near the Belgian town of Ypres, leaving thousands dead. In July 1917, again near Ypres, German artillery shells delivered a new kind of chemical agent — sulfur mustard. Between World War I and World War II, the debate about chemical warfare continued in the United States and in international forums. The Geneva Protocol was signed in 1925, prohibiting the “use in war of asphyxiating, poisonous or other gases, and of bacteriological methods of warfare.” But the Protocol did not address the production, storage or transfer of chemical weapons. In 1936, a German chemist searching for more potent insecticides accidentally discovered tabun, the fi rst nerve agent chemical weapon.