Swans: Their Biology and Natural History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Black Swans, Or the Limits of Statistical Modelling
Black swans, or the limits of statistical modelling Eric Marsden <[email protected]> Do I follow indicators that could tell me when my models may be wrong? On the 1001st day, a little before Thanksgiving, the turkey has a surprise. A turkey is fed for 1000 days. Each passing day confirms to its statistics department that the human race cares about its welfare “with increased statistical significance”. 2 / 58 A turkey is fed for 1000 days. Each passing day confirms to its statistics department that the human race cares about its welfare “with increased statistical significance”. On the 1001st day, a little before Thanksgiving, the turkey has a surprise. 2 / 58 Things that have never happened before, happen all the time. — Scott D. Sagan, The Limits of Safety: Organizations, ‘‘ Accidents, And Nuclear Weapons, 1993 In risk analysis, these are called “unexampled events” or “outliers” or “black swans”. 3 / 58 The term “black swan” was used in16th century discussions of impossibility (all swans known to Europeans were white). Explorers arriving in Australia discovered a species of swan that is black. The term is now used to refer to events that occur though they had been thought to be impossible. 4 / 58 Black swans ▷ Characteristics of a black swan event: • an outlier • lies outside the realm of regular expectations • nothing in the past can convincingly point to its possibility • carries an extreme impact ▷ Note that: • in spite of its outlier status, it is often easy to produce an explanation for the event after the fact • a black swan event may be a surprise for some, but not for others; it’s a subjective, knowledge-dependent notion • warnings about the event may have been ignored because of strong personal and organizational resistance to changing beliefs and procedures Source: The Black Swan: the Impact of the Highly Improbable, N. -

Bird Report 1999
YELLOWSTONE BIRD REPORT 1999 Terry McEneaney Yellowstone Center for Resources National Park Service Yellowstone National Park, Wyoming YCR–NR–2000–02 Suggested citation: McEneaney, T. 2000. Yellowstone Bird Report, 1999. National Park Service, Yellowstone Center for Resources, Yellowstone National Park, Wyoming, YCR–NR–2000–02. Cover: Special thanks to my wife, Karen McEneaney, for the stunning pencil drawing of a Golden Eagle (Aquila chrysaetos) talon. The Golden Eagle is one of Yellowstone’s most formidable avian predators. When viewing Golden Eagle talons up close, one soon realizes why the bird is a force to be reckoned with in the natural world. Title page: Great Horned Owlet. The photographs in this report are courtesy of Terry McEneaney. ii CONTENTS INTRODUCTION ..................................................................... 5 Bird Impression .............................................................. 20 Weather Patterns and Summary ....................................... 5 National Geographic Field Guide .................................... 21 THREATENED AND ENDANGERED SPECIES .............................. 7 Retirement of Yellowstone Pilot Dave Stradley .............. 21 Peregrine Falcon ............................................................... 7 Yellowstone Birds: Their Ecology and Distribution ....... 21 Bald Eagle ........................................................................ 7 Computerized Database ................................................. 21 Whooping Crane ............................................................. -

Whooper Swan)
Cygnus cygnus (Whooper Swan) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 5 Sources of reported national population data p. 8 Species factsheet bibliography p. 13 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Cygnus cygnus (Whooper Swan) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 Long-term -

Liste Provisoire 2012 Bateaux Modernes
21-sept-12 LISTE PROVISOIRE 2012 BATEAUX MODERNES BATEAU VOILE PLACE TCC LONG TYPE 1 ALBACOR IV FRA 1091 SAINT-TROPEZ 1.018 10.74 J 109 2 ALBACOR V FRA SNST 1.085 11.82 GRAND SOLEIL 39 3 ALIBI 2 FRA 35107 SNST 1.000 10.70 FIRST 36.7 4 ALPINA BY FINIMMO BVI 007 GRIMAUD 1.386 24.89 SWAN 80 5 ALTHINIMAX FRA 9533 SAINT-TROPEZ 1.020 10.74 J109 6 AMBRE MILES LAT 909 GRIMAUD 1.142 13.29 XP 44 7 ANDANTE II BEL 1308 GRIMAUD 1.095 12.92 GRAND SOLEIL 43 RACE 8 ARAGON NED 8313 SAINT-TROPEZ 1.464 22.00 MARTEN 72 9 ARGO FRA 21584 SNST 1.060 11.92 FIRST40.7 10 AROBAS FRA 60101 SAINT-TROPEZ 1.338 18.30 SWAN 601 11 ARRABIATA SUI 5400 SAINT-TROPEZ 1.148 16.27 GRAND SOLEIL 54 12 ART ROSE FRA 18067 SAINT-TROPEZ 1.128 10.40 WALLYNANO 2.60 13 ATTILA FRA 8304 SAINT-TROPEZ 0.956 10.11 DELHER 3/4 DB1TRA 14 AXELLE S FRA 29745 COGOLIN 1.173 13.83 SWAN 45 15 AZUREVE GER 6102 COGOLIN 1.031 12.35 HANSE 411 16 BALLYTRIM GBR 5046R GRIMAUD 1.076 14.05 SWAN 46 17 BAM BAM IV GBR 1071L GRIMAUD 1.163 14.98 FIRST 50 18 BELLA DONNA ITA 900 GRIMAUD 1.197 14.00 V46 19 BERNINA FRA 34788 SAINT-TROPEZ 0.955 09.48 FIRST 31.7 20 BISOU SALE FRA 38084 SNST 0.990 09.46 FIRST 30 21 CACHOU FRA 35969 SNST 1.145 12.80 SLY 42 22 CALAGAN FRA 29198 GRIMAUD 1.052 11.37 IMX 38 23 CHELSEA GBR 9162T GRIMAUD 1.004 10.70 FIRST36.7 24 COYOTE RUS 7 GRIMAUD 1.155 13.72 IMX 45 25 CREME ANGLAISE GBR2826L SAINTE MAXIME 0.996 10.39 FIRST 30 26 D DREAMS FRA 43 509 GRIMAUD 1.096 16.00 HANSE 54 27 DARKICE GBR 34R GRIMAUD 1.280 13.68 FRERS 44 28 DAY BY DAY ITA 204 GRIMAUD 1.053 11.60 X 35 29 DISCO INFERNO -

2014 Newport Bermuda Race® Spirit of Tradition Class 0 St. David's
2014 Newport Bermuda Race® Official Class and Division Results Scored Under ORR blank Spirit of Tradition Class 0 Cls Div Finish Elapsed Corrected PosPosSail No Yacht Name Design/Model Captain/Owner Affiliation Status Time(EDT) Time Time BER 3 Mast Bermuda Sloop 06/26 0 0 SPIRIT OF BERMUDA None 132:59:23 00:00:00 688 Schooner Foundation 01:59:23 blank St. David's Lighthouse Division Class 1 Cls Div Finish Elapsed Corrected PosPosSail No Yacht Name Design/Model Captain/Owner Affiliation Status Time(EDT) Time Time USA Hinckley 06/25 1 1 ACTAEA Michael M Cone CCA, CYCOP 121:44:39 80:25:58 3815 B40 14:54:39 USA Douglas R. 06/25 2 2 FLYER Cal 40 TAYC / MRYC 121:18:16 81:05:50 2213 Abbott 14:28:16 USA 06/25 3 3 SINN FEIN Cal 40 Peter S. Rebovich RYC 120:48:38 81:10:53 1818 13:58:38 USA Geoffrey M. 06/25 4 5 GADZOOKS C&C 38 Norwalk YC 122:13:57 82:50:51 21108 Beringer 15:23:57 USA Andrew F 06/25 5 7 AURORA Tartan 41 CYC 117:59:08 83:11:26 14111 Kallfelz 11:09:08 USA Swan 43 CCA, Stamford, 06/25 6 8 HIRO MARU Hiroshi Nakajima 119:49:17 83:28:13 32510 Classic NYYC 12:59:17 VanTol, USA C&C 06/25 7 14 ELIMINATOR Paul;VandeVusse,Bayview YC 122:21:24 84:20:48 15370 35MKII 15:31:24 Bruce USA 06/25 8 20 GLIM CC 40 William R. -

April 2018 VOL XXVIII No. 1
TRUMPETINGS Voice of The Trumpeter Swan Society 12615 Rockford Rd., Plymouth, MN 55441-1248 715-441-1994 www.trumpeterswansociety.org [email protected] Since 1968: Assuring the vitality and welfare of wild Trumpeter Swans VOL. XXVIII No. 1 APRIL 2018 Changes to Migratory Bird Treaty Act weaken bird protections The Migratory Bird Treaty Act (MBTA), signed into law in 1918, is among the oldest and most effective wildlife protection laws on the books. When Congress passed the MBTA in 1918, it codified a treaty already signed with Canada, then part of Great Britain. The Treaty was in response to the serious overharvest of numerous bird species that had resulted in extinction in a few instances and near extinction in some species. Since 1918, the MBTA has broadened its international scope through treaties with Mexico, Japan, and Russia. The MBTA is credited with saving numerous species from extinction, including Trumpeter Swans. It continues to protect nearly all native birds in the U.S. covering more than 1,000 species, including Trumpeter Swans. The Department of Interior made significant changes to the Legislation in Congress (HR 4239), and a new interpretation interpretation of the century-old Migratory Bird Treaty Act. Photo by Margaret Smith of the law by the Administration, would end the ability to hold industries accountable for bird deaths. Industries would only be held accountable if their intention or purpose was to harm birds through their activities. This rolls back decades of bipartisan support and interpretation of the MBTA. It also removes industry incentives to prevent bird deaths and its associated penalties. -

C35-Echevarria-Et-Al
Cotinga 35 Nesting biology of Coscoroba Swan Coscoroba coscoroba at La Angostura Dam, Tafí del Valle, Tucumán, Argentina Ada Lilian Echevarria, María Constanza Cocimano, José María Chani and Claudia Fabiana Marano Received 6 July 2011; final revision accepted 27 June 2012 Cotinga 35 (2013): 13–16 El Cisne Coscoroba Coscoroba coscoroba nidifica en latitudes mayores a 33ºS desde Buenos Aires (Argentina) y Chiloé (Chile) hasta Isla Grande en Tierra del Fuego y, ocasionalmente, en las islas Malvinas. Mostramos el primer registro de nidificación a 26º55’06”S 65º41’36”O y 2.000 msnm. Nuestro objetivo fue estudiar la nidificación de C. coscoroba para contribuir con información sobre la reproducción de esta especie. Llevamos a cabo nuestro estudio desde agosto de 2004 a noviembre de 2005 en el dique La Angostura, Argentina. Registramos 26 individuos e identificamos ocho nidos, cinco de los cuales mostraron actividad, con un total de cinco puestas. One of two species of swans in South America9, designated by a number. We visited each nest once Coscoroba Swan Coscoroba coscoroba inhabits per week and recorded data on materials and nest shallow brackish lagoons with abundant fringing and egg measurements. In addition, we recorded vegetation3. Documented nesting records outside nest activity (adults in the environs of nests and the main breeding area are scarce8. It nests at nest stage) during each visit. We spent 30 minutes latitudes above 33ºS from Buenos Aires (Argentina) observing each nest during each weekly visit p.a., and Chiloé (Chile) to Isla Grande in Tierra del with a total of 1,140 minutes of observation. -

ON 23(4) 555-567.Pdf
ORNITOLOGIA NEOTROPICAL 23: 555–567, 2012 © The Neotropical Ornithological Society REPRODUCTIVE BIOLOGY AND PAIR BEHAVIOR DURING INCUBATION OF THE BLACK-NECKED SWAN (CYGNUS MELANOCORYPHUS) Carmen Paz Silva1, Roberto Pablo Schlatter2 & Mauricio Soto-Gamboa1 1Instituto de Ciencias Ambientales y Evolutivas, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile. E-mail: [email protected] 2Instituto de Ciencias Marinas y Limnológicas, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile. Resumen. – Biología reproductiva y comportamiento del Cisne de Cuello Negro (Cygnus melano- coryphus). – El cisne de cuello negro (Cygnus melanocoryphus) es la única especie del género Cygnus que habita en Sudamérica. Si bien, el resto de las especies del género han sido extensamente estudia- das, para C. melanocoryphus aún no se ha descrito la mayor parte de su biología reproductiva. En este estudio evaluamos el comienzo y extensión de la temporada reproductiva, y el tamaño de nidada utili- zando datos recolectados en el Santuario Carlos Anwandter (SCA), Valdivia, Chile; correspondientes a 18 temporadas reproductivas. Por otro lado, estudiamos el comportamiento de las parejas durante el periodo de incubación, durante una temporada reproductiva. Se estimó el presupuesto de tiempo y dis- tancia con respecto al nido en seis parejas nidificantes. El periodo de nidificación se puede extender desde junio a enero y está influenciado por el tamaño de la población reproductiva y las condiciones cli- máticas. El tamaño de nidada se mantiene constante con una moda de tres huevos (promedio 3.13 ± 0.017; n = 5897). Ambos miembros de la pareja desarrollan actividades de vigilancia y mantención del nido, sin embargo, la incubación es una tarea exclusiva de la hembra, mientras que la defensa activa del nido es realizada por el macho. -
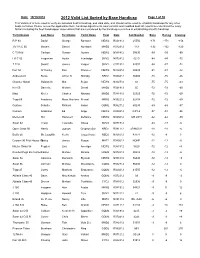
2012 Valid List Sorted by Base Handicap
Date: 10/19/2012 2012 Valid List Sorted by Base Handicap Page 1 of 30 This Valid List is to be used to verify an individual boat's handicap, and valid date, and should not be used to establish handicaps for any other boats not listed. Please review the appilication form, handicap adjustments, boat variants and modified boat list reports to understand the many factors including the fleet handicapper observations that are considered by the handicap committee in establishing a boat's handicap Yacht Design Last Name First Name Yacht Name Fleet Date Sail Number Base Racing Cruising R P 90 David George Rambler NEW2 R021912 25556 -171 -171 -156 J/V I R C 66 Meyers Daniel Numbers MHD2 R012912 119 -132 -132 -120 C T M 66 Carlson Gustav Aurora NEW2 N081412 50095 -99 -99 -90 I R C 52 Fragomen Austin Interlodge SMV2 N072412 5210 -84 -84 -72 T P 52 Swartz James Vesper SMV2 C071912 52007 -84 -87 -72 Farr 50 O' Hanley Ron Privateer NEW2 N072412 50009 -81 -81 -72 Andrews 68 Burke Arthur D Shindig NBD2 R060412 55655 -75 -75 -66 Chantier Naval Goldsmith Mat Sejaa NEW2 N042712 03 -75 -75 -63 Ker 55 Damelio Michael Denali MHD2 R031912 55 -72 -72 -60 Maxi Kiefer Charles Nirvana MHD2 R041812 32323 -72 -72 -60 Tripp 65 Academy Mass Maritime Prevail MRN2 N032212 62408 -72 -72 -60 Custom Schotte Richard Isobel GOM2 R062712 60295 -69 -69 -57 Custom Anderson Ed Angel NEW2 R020312 CAY-2 -57 -51 -36 Merlen 49 Hill Hammett Defiance NEW2 N020812 IVB 4915 -42 -42 -30 Swan 62 Tharp Twanette Glisse SMV2 N071912 -24 -18 -6 Open Class 50 Harris Joseph Gryphon Soloz NBD2 -

Whooper Swan
Whooper Swan The Whooper Swan (Cygnus cygnus), pronounced hooper swan, is a large Northern Hemisphere swan. It is the Eurasian counterpart of the North American Trumpeter Swan, and the type species for the genus Cygnus. Francis Willughby and John Ray's Ornithology of 1676 referred to this swan as "the Elk, Hooper, or wild Swan". The scientific name is from cygnus, the Latin for "swan". The Whooper Swan is similar in appearance to the Bewick's Swan. It is larger, however, at a length of 140–165 cm (55–65 in) and a wingspan of 205–275 cm (81–108 in). It is considered to be amongst the heaviest flying birds. It has a more angular head shape and a more variable bill pattern that always shows more yellow than black (Bewick's Swans have more black than yellow). Like their close relatives, Whooper Swans are vocal birds with a call similar to the Trumpeter Swan. Despite their size Whooper Swans are powerful fliers and can migrate hundreds or even thousands of miles to their wintering sites in southern Europe and eastern Asia. They breed in subarctic Eurasia, further south than Bewick’s in the taiga zone. They are rare breeders in northern Scotland, particularly in Orkney, and no more than five pairs have bred there in recent years; a handful of pairs have also bred in Ireland in recent years. This bird is an occasional vagrant to the Indian Subcontinent and western North America. Icelandic breeders overwinter in the United Kingdom and Ireland, especially in the wildfowl nature reserves of the RSPB and of the Wildfowl and Wetlands Trust. -
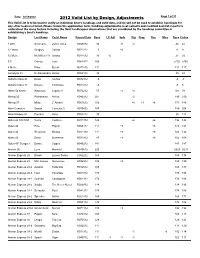
Valid List by Design
Date: 10/19/2012 2012 Valid List by Design, Adjustments Page 1 of 31 This Valid List is to be used to verify an individual boat's handicap, and valid date, and should not be used to establish handicaps for any other boats not listed. Please review the appilication form, handicap adjustments, boat variants and modified boat list reports to understand the many factors including the fleet handicapper observations that are considered by the handicap committee in establishing a boat's handicap. Design Last Name Yacht Name Record Date Base LP Adj Spin Rig Prop Rec Misc Race Cruise 1 D35 Schimenti Zefiro Toma R043012 36 -9 -3 24 42 12 Metre Gregory Valiant R071212 3 +6 9 9 12 Metre Mc Millen 111 Onawa R011512 33 -6 27 33 5.5 Carney Lyric R082912 156 u156 u165 8 Metre Palm Quest N071612 111 111 117 Aerodyne 38 D' Alessandro Alexis R053112 39 39 48 Akilaria Class 40 Davis Amhas R072312 -9 -9 -3 Akilaria Class 40 Dreese Toothface R041012 -9 -9 0 Alben 54 Ketch Wiseman Legacy V R070212 57 +6 +6 69 78 Alberg 35 Prefontaine Helios R042312 201 -3 198 210 Alberg 37 Mintz L' Amarre R061612 156 +6 +3 +6 171 186 Albin Cumulus Droste Cumulus 3 R030412 189 189 204 Albin Nimbus 42 Pomfret Anne R052212 99 99 111 Alden 42 S D S M Vieira Cadence R011312 120 +6 +6 132 144 Alden 44 Rice Pilgrim N053112 111 +9 +6 126 141 Alden 44 Weisman Nostos R011312 111 +9 +6 126 132 Alden 45 Davin Querence R071912 87 +9 +6 102 108 Alden 45" Seagoer Dunne Cygnet N040212 141 141 147 Alerion 26 Lurie Mischief R040612 225 U225 U231 Alerion Express 28 Brown Lumen Solare C082212 -

Wingps 5 Voyager
Polairdiagrammen -Squib ALBIN ALPHA Auklet 9 Bavaria 33cr Bavaria 42 Bianca III 1 Ton Albin Ballad AVANCE 24 Bavaria 34 1.85 Bavaria 42cruiser BIRDIE 32 1-Tonner OO Albin Balled Avance 36 Bavaria 34 AC Bavaria 430 lagoon Blue Moon 8 mtr. 100D 50 ALBIN DELTA B 26 BAVARIA 34 CRUISER Bavaria 44 1.65 Blusail 24 116 Jezquel Albin Nova B 31 Bavaria 34 Bavaria 44 AC 03-0 bno 183 11_Metre Albin Singoalla B&C 41 BAVARIA 340 C Bavaria 44 Vision BOLING 1D35 ALBIN STRATUS B&C IMS37CR Bavaria 340 x 1.70 Bavaria 44 BONGO 870 1D48 ALBIN VEGA 27 B&C46 Bavaria 34_3x1.35 Bavaria 44x1.95 BONGO 9.60 1_2 TON ONE OFF ALBIN VIGGEN B-32 Bavaria 35 exlc. Bavaria 46 2.00 BONIN 358 1_2 Ton ALC 46 BA 40 BAVARIA 35 HOLIDAY BAVARIA 46 C Bonita 767 1_2 Tonner ALEKSTAR 25 BAD 27 Bavaria 35 Holyday BAVARIA 46 CR Bonita767x1.40 1_4 TON ONE OFF Alligator BAD 37 Bavaria 35 Match D BAVARIA 46 CRUISER Bood 28 1_4 Ton ALO 28 Bahama 43 Bavaria 35 match BAVARIA 46 HOLIDAY Bood 36 2 TONNER Aloa 27 Sport BAKKE 26 BAVARIA 350 Bavaria 46 x 2.00 Booty 24 312 PLUS ALOA 27 BALLAD Bavaria 36 AC 2003 BAVARIA 46 Bosgraaf 37x1.9 50 ‘ IOR ALPA 12.70 Baltic 35 Bavaria 36 AC 98-9 BAVARIA 47 BOXER 24 7 m S ALPA 34 Baltic 37 x2.10 Bavaria 36 AC BAVARIA 50 Brabant II 717 ALPA SUPERMAICA Baltic 37 BAVARIA 36 C Bavaria 50x2.0 BRABANT 747 ALU 41 Baltic 37x2.06 Bavaria 36 CR 01-0 BAVARIA 707 BRAMADOR 34 8 M ALU 980 Baltic 38 BAVARIA 36 CRUISER Bavaria 820x1.30 Breehoorn37x1.90 8 Metres JI Alu.