Hygiene Defense Behaviors Used by a Fungus-Growing Ant Depend on the Fungal Pathogen Stages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A New Species of Trachymyrmex (Hymenoptera, Formicidae) Fungus-Growing Ant from the Sierra Madre Oriental of Northeastern Mexico
A peer-reviewed open-access journal ZooKeys 706:A 73–94 new (2017)species of Trachymyrmex (Hymenoptera, Formicidae) fungus-growing ant... 73 doi: 10.3897/zookeys.706.12539 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Trachymyrmex (Hymenoptera, Formicidae) fungus-growing ant from the Sierra Madre Oriental of northeastern Mexico Sergio R. Sánchez-Peña1, Manuela Citlali Chacón-Cardosa2, Ricardo Canales-del-Castillo2, Lauren Ward3, Diana Resendez-Pérez2 1 Departamento de Parasitología, Universidad Autónoma Agraria Antonio Narro, Calzada Antonio Narro 1923, Saltillo, Coahuila, México C.P. 25315 2 Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas, Av. Universidad S/N, Cd. Universitaria, San Nicolás de los Garza, Nuevo León 66455 3 Department of Entomo- logy, Texas A&M University, TAMU 2475 College Station, TX 77843-2475, USA Corresponding author: Diana Resendez-Pérez ([email protected]) Academic editor: Brian Fisher | Received 4 March 2017 | Accepted 22 August 2017 | Published 4 October 2017 http://zoobank.org/4849ABEF-71BE-4398-8317-1D329A3018E0 Citation: Sánchez-Peña SR, Chacón-Cardosa MC, Canales-del-Castillo R, Ward L, Resendez-Pérez D (2017) A new species of Trachymyrmex (Hymenoptera, Formicidae) fungus-growing ant from the Sierra Madre Oriental of northeastern Mexico. ZooKeys 706: 73–94. https://doi.org/10.3897/zookeys.706.12539 Abstract Here we describe a new species of Trachymyrmex, T. pakawa sp. n., from the Gran Sierra Plegada range of the Sierra Madre Oriental, in the states of Coahuila and Nuevo Leon, northeastern Mexico. Trachymyrmex pakawa is a large-sized species compared to other North American Trachymyrmex. -

Digging Deeper Into the Ecology of Subterranean Ants: Diversity and Niche Partitioning Across Two Continents
diversity Article Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents Mickal Houadria * and Florian Menzel Institute of Organismic and Molecular Evolution, Johannes-Gutenberg-University Mainz, Hanns-Dieter-Hüsch-Weg 15, 55128 Mainz, Germany; [email protected] * Correspondence: [email protected] Abstract: Soil fauna is generally understudied compared to above-ground arthropods, and ants are no exception. Here, we compared a primary and a secondary forest each on two continents using four different sampling methods. Winkler sampling, pitfalls, and four types of above- and below-ground baits (dead, crushed insects; melezitose; living termites; living mealworms/grasshoppers) were applied on four plots (4 × 4 grid points) on each site. Although less diverse than Winkler samples and pitfalls, subterranean baits provided a remarkable ant community. Our baiting system provided a large dataset to systematically quantify strata and dietary specialisation in tropical rainforest ants. Compared to above-ground baits, 10–28% of the species at subterranean baits were overall more common (or unique to) below ground, indicating a fauna that was truly specialised to this stratum. Species turnover was particularly high in the primary forests, both concerning above-ground and subterranean baits and between grid points within a site. This suggests that secondary forests are more impoverished, especially concerning their subterranean fauna. Although subterranean ants rarely displayed specific preferences for a bait type, they were in general more specialised than above-ground ants; this was true for entire communities, but also for the same species if they foraged in both strata. Citation: Houadria, M.; Menzel, F. -

Ant–Fungus Species Combinations Engineer Physiological Activity Of
© 2014. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2014) 217, 2540-2547 doi:10.1242/jeb.098483 RESEARCH ARTICLE Ant–fungus species combinations engineer physiological activity of fungus gardens J. N. Seal1,2,*, M. Schiøtt3 and U. G. Mueller2 ABSTRACT such complexity, the fungus-gardening insects have evolved obligate Fungus-gardening insects are among the most complex organisms macro-symbioses with specific clades of fungi, and use fungal because of their extensive co-evolutionary histories with obligate symbionts essentially as an external digestive organ that allows the fungal symbionts and other microbes. Some fungus-gardening insect insect to thrive on otherwise non-digestible substrates, such as lineages share fungal symbionts with other members of their lineage structural carbohydrates of plants (e.g. cellulose) (Aanen et al., and thus exhibit diffuse co-evolutionary relationships, while others 2002; Aylward et al., 2012a; Aylward et al., 2012b; Bacci et al., exhibit little or no symbiont sharing, resulting in host–fungus fidelity. 1995; Farrell et al., 2001; De Fine Licht and Biedermann, 2012; The mechanisms that maintain this symbiont fidelity are currently Martin, 1987a; Mueller et al., 2005). One of the most striking unknown. Prior work suggested that derived leaf-cutting ants in the attributes of these symbioses is the degree of physiological genus Atta interact synergistically with leaf-cutter fungi (Attamyces) integration: the insect host functions as a distributor of fungal by exhibiting higher fungal growth rates and enzymatic activities than enzymes, which digest plant fibers external to the insect’s body when growing a fungus from the sister-clade to Attamyces (so-called (Aanen and Eggleton, 2005; Aylward et al., 2012b; De Fine Licht ‘Trachymyces’), grown primarily by the non-leaf cutting and Biedermann, 2012; De Fine Licht et al., 2013; Martin, 1987b; Trachymyrmex ants that form, correspondingly, the sister-clade to Schiøtt et al., 2010). -

Trachymyrmex Septentrionalis) During and After a Record Drought
Insect Conservation and Diversity (2010) 3, 134–142 doi: 10.1111/j.1752-4598.2010.00085.x Distribution of the fungus-gardening ant (Trachymyrmex septentrionalis) during and after a record drought JON N. SEAL and WALTER R. TSCHINKEL Department of Biological Science, Florida State University, Tallahassee, FL 32306, USA Abstract. 1. Insects are known to be influenced by global climate change, especially by drought and increased temperatures. 2. Although ants are widely regarded to be indicator or keystone species and eco- system engineers, we do not know how ants may respond to global climate change. 3. This study reports the range contraction of an extremely abundant fungus-gar- dening ant (Trachymyrmex septentrionalis) over a 3-year period that coincided with the end of a record drought in southeastern North America. 4. Reduction in nest number appears to be the result of an increase in water-table levels and decrease in soil aridity. 5. Therefore T. septentrionalis should be expected to increase its abundance and presumably its ecological impact during multiyear droughts. Key words. Attini, climate change, El Nin˜o, ENSO, global warming, La Nin˜a, nest architecture, Pinus palustris, Quercus laevis, water table. Introduction on other insect taxa. Ants are widely regarded as keystone mem- bers and ecosystem engineers in many ecosystems because of Global warming and changing of hydrological regimes are their known contributions to ecoystem processes (Andersen, thought to have profound consequences on the distribution and 1997; Agosti et al., 2000; MacMahon et al., 2000; Jime´nez & abundance of ectotherms such as insects (Chown & Nicholson, Decae¨ns, 2006; Jouquet et al., 2007; Cammeraat & Risch, 2008; 2004). -

Borowiec Et Al-2020 Ants – Phylogeny and Classification
A Ants: Phylogeny and 1758 when the Swedish botanist Carl von Linné Classification published the tenth edition of his catalog of all plant and animal species known at the time. Marek L. Borowiec1, Corrie S. Moreau2 and Among the approximately 4,200 animals that he Christian Rabeling3 included were 17 species of ants. The succeeding 1University of Idaho, Moscow, ID, USA two and a half centuries have seen tremendous 2Departments of Entomology and Ecology & progress in the theory and practice of biological Evolutionary Biology, Cornell University, Ithaca, classification. Here we provide a summary of the NY, USA current state of phylogenetic and systematic 3Social Insect Research Group, Arizona State research on the ants. University, Tempe, AZ, USA Ants Within the Hymenoptera Tree of Ants are the most ubiquitous and ecologically Life dominant insects on the face of our Earth. This is believed to be due in large part to the cooperation Ants belong to the order Hymenoptera, which also allowed by their sociality. At the time of writing, includes wasps and bees. ▶ Eusociality, or true about 13,500 ant species are described and sociality, evolved multiple times within the named, classified into 334 genera that make up order, with ants as by far the most widespread, 17 subfamilies (Fig. 1). This diversity makes the abundant, and species-rich lineage of eusocial ants the world’s by far the most speciose group of animals. Within the Hymenoptera, ants are part eusocial insects, but ants are not only diverse in of the ▶ Aculeata, the clade in which the ovipos- terms of numbers of species. -

Hymenoptera: Formicidae), and Generalized Ant and Arthropod Diversity
COMMUNITY AND ECOSYSTEM ECOLOGY Positive Association Between Densities of the Red Imported Fire Ant, Solenopsis invicta (Hymenoptera: Formicidae), and Generalized Ant and Arthropod Diversity LLOYD W. MORRISON AND SANFORD D. PORTER Center for Medical, Agricultural and Veterinary Entomology, USDAÐARS, P.O. Box 14565, Gainesville, FL 32604 Environ. Entomol. 32(3): 548Ð554 (2003) ABSTRACT The invasive ant, Solenopsis invicta Buren, is a threat to native arthropod biodiversity. We compared areas with naturally varying densities of mostly monogyne S. invicta and examined the association of S. invicta density with three diversity variables: (1) the species richness of ants, (2) the species richness of non-ant arthropods, and (3) the abundance of non-S. invicta ants. Pitfall traps were used to quantify S. invicta density and the three diversity variables; measurement of mound areas provided a complementary measure of S. invicta density. We sampled 45 sites of similar habitat in north central Florida in both the spring and autumn of 2000. We used partial correlations to elucidate the association between S. invicta density and the three diversity variables, extracting the effects of temperature and humidity on foraging activity. Surprisingly, we found moderate positive correlations between S. invicta density and species richness of both ants and non-ant arthropods. Weaker, but usually positive, correlations were found between S. invicta density and the abundance of non-S. invicta ants. A total of 37 ant species, representing 16 genera, were found to coexist with S. invicta over the 45 sites. These results suggest that S. invicta densities as well as the diversities of other ants and arthropods are regulated by common factors (e.g., productivity). -

Downloaded from Brill.Com09/30/2021 01:18:19AM Via Free Access 10 Tijdschrift Voor Entomologie, Volume 155, 2012
Tijdschrift voor Entomologie 155 (2012) 9–14 brill.nl/tve Two new species of the genus Myrmica (Hymenoptera: Formicidae: Myrmicinae) from the Himalaya Himender Bharti Two new species of the genus Myrmica are described from the Himalaya. Myrmica adrijae sp. n. is reported from North-western region in India, while Myrmica pseudorugosa sp. n. is reported from North-eastern Pakistan. Myrmica adrijae sp. n. and Myrmica pseudorugosa sp. n. belong to the smythiesii and rugosa species groups respectively. Both species are considerably distinct from already described species in these groups. Himender Bharti, Department of Zoology and Environmental Sciences, Punjabi University Patiala, India, 147002. [email protected] Introduction lided with the Eurasian plate, followed by a sec- The genus Myrmica Latreille, 1804 is represented ond phase of mountain development about 65 mil- in the old world by 146 valid species, which are lion years ago. Rising of the Himalaya as an isola- well distributed in the Palearctic zone and in south- tion barrier has led to a high degree of endemism east Asian tropical and subtropical regions (Rad- (Radchenko & Elmes 2001, 2010; Bharti 2008a, chenko & Elmes 2010; Bharti 2011, 2012; Bharti 2008b, 2011, 2012; Bharti & Sharma 2011a, 2011b, & Sharma 2011a, 2011b, 2011c). The central Asian 2011c). Only during the last ten years, the author has mountains, which comprise Hindu Kush, Karako- started exploring the Himalayan fauna. The region rum and the south-western slopes of the Himalaya appears to have quite a number of undescribed or (Afghanistan, Pakistan, India, Nepal and Bhutan), unnoticed species which would contribute in under- harbour 37 species representing seven species groups. -

Description of a New Genus of Primitive Ants from Canadian Amber
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 8-11-2017 Description of a new genus of primitive ants from Canadian amber, with the study of relationships between stem- and crown-group ants (Hymenoptera: Formicidae) Leonid H. Borysenko Canadian National Collection of Insects, Arachnids and Nematodes, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/insectamundi Part of the Ecology and Evolutionary Biology Commons, and the Entomology Commons Borysenko, Leonid H., "Description of a new genus of primitive ants from Canadian amber, with the study of relationships between stem- and crown-group ants (Hymenoptera: Formicidae)" (2017). Insecta Mundi. 1067. http://digitalcommons.unl.edu/insectamundi/1067 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0570 Description of a new genus of primitive ants from Canadian amber, with the study of relationships between stem- and crown-group ants (Hymenoptera: Formicidae) Leonid H. Borysenko Canadian National Collection of Insects, Arachnids and Nematodes AAFC, K.W. Neatby Building 960 Carling Ave., Ottawa, K1A 0C6, Canada Date of Issue: August 11, 2017 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Leonid H. Borysenko Description of a new genus of primitive ants from Canadian amber, with the study of relationships between stem- and crown-group ants (Hymenoptera: Formicidae) Insecta Mundi 0570: 1–57 ZooBank Registered: urn:lsid:zoobank.org:pub:C6CCDDD5-9D09-4E8B-B056-A8095AA1367D Published in 2017 by Center for Systematic Entomology, Inc. -

Zootaxa 2878: 1–61 (2011) ISSN 1175-5326 (Print Edition) Monograph ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (Online Edition)
Zootaxa 2878: 1–61 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 2878 Generic Synopsis of the Formicidae of Vietnam (Insecta: Hymenoptera), Part I — Myrmicinae and Pseudomyrmecinae KATSUYUKI EGUCHI1, BUI TUAN VIET2 & SEIKI YAMANE3 1Department of International Health, Institute of Tropical Medicine, Nagasaki University, Nagasaki 852-8523, Japan. E-mail: [email protected] 2Vietnam National Museum of Nature, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam. E-mail: [email protected] 3Department of Earth and Environmental Sciences, Faculty of Science, Kagoshima University, Kagoshima 890-0065, Japan. Magnolia Press Auckland, New Zealand Accepted by J. Longino: 25 Jan. 2011; published: 13 May 2011 KATSUYUKI EGUCHI, BUI TUAN VIET & SEIKI YAMANE Generic Synopsis of the Formicidae of Vietnam (Insecta: Hymenoptera), Part I — Myrmicinae and Pseudomyrmecinae (Zootaxa 2878) 61 pp.; 30 cm. 13 May 2011 ISBN 978-1-86977-667-1 (paperback) ISBN 978-1-86977-668-8 (Online edition) FIRST PUBLISHED IN 2011 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2011 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 2878 © 2011 Magnolia Press EGUCHI ET AL. -
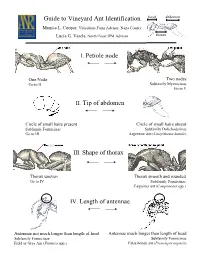
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

The Venom Apparatus and Other Morphological Characters of the Ant Martialis Heureka (Hymenoptera, Formicidae, Martialinae)
Volume 50(26):413‑423, 2010 The venom apparaTus and oTher morphological characTers of The anT Martialis heureka (hymenopTera, formicidae, marTialinae) carlos roberTo ferrreira brandão1,3 Jorge luis machado diniz2 rodrigo dos sanTos machado feiTosa1 AbstrAct We describe and illustrate the venom apparatus and other morphological characters of the recently described Martialis heureka ant worker, a supposedly specialized subterranean predator which could be the sole surviving representative of a highly divergent lineage that arose near the dawn of ant diversification. M. heureka was described as the single species of a genus in the subfamily, Martialinae Rabeling and Verhaagh, known from a single worker. However because the authors had available a unique specimen, dissections and scanning electron microscopy from coated specimens were not possible. We base our study on two worker individuals collected in Manaus, AM, Brazil in 1998 and maintained in 70% alcohol since then; the ants were partially destroyed because of desiccation during transport to São Paulo and subsequent efforts to rescue them from the vial. We were able to recover two left mandibles, two pronota, one dismembered fore coxa, one meso-metapropodeal complex with the median and hind coxae and trochanters still attached, one postpetiole, two gastric tergites, the pygidium and the almost complete venom apparatus (lacking the gonostylus and anal plate). We illustrate and describe the pieces, and compare M. heureka worker morphology with other basal ant subfamilies, concluding it does merit subfamilial status. Keywords: Ant phylogeny; Formicidae; Martialis; Ultrastructure; Venom Apparatus. IntroductIon dusk in a primary lowland rainforest walking on the leaf litter. The ant Martialis heureka was recently described The phylogenetic position of this ant was inferred by Rabeling and Verhaagh (in Rabeling et al. -

Newly Discovered Sister Lineage Sheds Light on Early Ant Evolution
Newly discovered sister lineage sheds light on early ant evolution Christian Rabeling†‡§, Jeremy M. Brown†¶, and Manfred Verhaagh‡ †Section of Integrative Biology, and ¶Center for Computational Biology and Bioinformatics, University of Texas, 1 University Station C0930, Austin, TX 78712; and ‡Staatliches Museum fu¨r Naturkunde Karlsruhe, Erbprinzenstr. 13, D-76133 Karlsruhe, Germany Edited by Bert Ho¨lldobler, Arizona State University, Tempe, AZ, and approved August 4, 2008 (received for review June 27, 2008) Ants are the world’s most conspicuous and important eusocial insects and their diversity, abundance, and extreme behavioral specializations make them a model system for several disciplines within the biological sciences. Here, we report the discovery of a new ant that appears to represent the sister lineage to all extant ants (Hymenoptera: Formicidae). The phylogenetic position of this cryptic predator from the soils of the Amazon rainforest was inferred from several nuclear genes, sequenced from a single leg. Martialis heureka (gen. et sp. nov.) also constitutes the sole representative of a new, morphologically distinct subfamily of ants, the Martialinae (subfam. nov.). Our analyses have reduced the likelihood of long-branch attraction artifacts that have trou- bled previous phylogenetic studies of early-diverging ants and therefore solidify the emerging view that the most basal extant ant lineages are cryptic, hypogaeic foragers. On the basis of morpho- logical and phylogenetic evidence we suggest that these special- EVOLUTION ized subterranean predators are the sole surviving representatives of a highly divergent lineage that arose near the dawn of ant diversification and have persisted in ecologically stable environ- ments like tropical soils over great spans of time.