Red Blood Cell Rheology in Sepsis K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Canine Immune-Mediated Hemolytic Anemia – Brief Review
TRADITION AND MODERNITY IN VETERINARY MEDICINE, 2018, vol. 3, No 1(4): 59–64 CANINE IMMUNE-MEDIATED HEMOLYTIC ANEMIA – BRIEF REVIEW Iliyan Manev1, Victoria Marincheva2 1University of Forestry, Faculty of Veterinary Medicine, Sofia, Bulgaria 2Animal Rescue, Sofia, Bulgaria E-mail: [email protected] ABSTRACT Immune-mediated hemolytic anemia (IMHA) is a common autoimmune disorder in dogs. It affects both sexes but occurs more often in female, middle-aged animals. IMHA can be idiopathic (primary) or secondary to infectious, neoplastic and autoimmune disorders. There is an acute regenerative anemia with accompanying hypoxia. Destruction of erythrocytes can be intravascular (as a result of complement system activation) or extravascular (removal of antibody-coated red blood cells by the macrophages in the liver and spleen). Diag- nosis is based on the presence of anemia, in vitro autoagglutination, positive direct antiglobulin test (Coomb`s test), detection of spherocytes. It is crucial to exclude possible secondary causes. The treatment protocol aims to cease cell destruction by high doses of corticosteroids, aggressive supportive care and long-term application of immunosuppressive drug combinations. Still lethality is high because of complications (pulmonary throm- boembolism, DIC), medication resistance, relapses. Key words: immune-mediated, anemia, canine, hemolysis, immunosuppressive drugs. Immune-mediated hemolytic anemia is one of the commonly diagnosed canine autoimmune diseases and a model of acute and clinically relevant anemia. Impaired -

Canine Multiple Myeloma
Canine Multiple Myeloma Meredith Maczuzak, DVM; Kenneth S. Latimer, DVM, PhD; Paula M. Krimer, DVM, DVSc; and Perry J. Bain, DVM, PhD Class of 2003 (Maczuzak) and Department of Pathology (Latimer, Krimer, Bain), College of Veterinary Medicine, University of Georgia, Athens, GA 30602-7388 Introduction Multiple myeloma or plasma cell myeloma, is a neoplasm of well- differentiated B cell lymphocytes typically originating from the bone marrow. Neoplastic cells can metastasize widely, having a predilection for bone and resulting in osteolysis. The malignant transformation of a single B cell can secrete a homogenous immunoglobulin product known as paraprotein, which will mimic the structure of normal immunoglobulins. Overabundant production of paraprotein, consisting of any of the immunoglobulin classes, will appear as a sharp, well-defined peak or monoclonal gammopathy on serum electrophoresis. The most frequently encountered multiple myelomas secrete IgG or IgA paraproteins, however IgM myelomas (macroglobulinemia) have also been diagnosed in companion animals. Light chain disease is caused by plasma cell overproduction of the light chain segment of the immunoglobulin complex, consisting of either the lambda or kappa light chain. These proteins are referred to as Bence-Jones proteins and are the most commonly observed immunoglobulin fragments in the monoclonal gammopathies.2 There are rare instances where a malignant plasma cell neoplasm will be nonsecretory. These tumors occur in approximately 1% of all cases of multiple myeloma and are referred -
Complete Blood Count in Primary Care
Complete Blood Count in Primary Care bpac nz better medicine Editorial Team bpacnz Tony Fraser 10 George Street Professor Murray Tilyard PO Box 6032, Dunedin Clinical Advisory Group phone 03 477 5418 Dr Dave Colquhoun Michele Cray free fax 0800 bpac nz Dr Rosemary Ikram www.bpac.org.nz Dr Peter Jensen Dr Cam Kyle Dr Chris Leathart Dr Lynn McBain Associate Professor Jim Reid Dr David Reith Professor Murray Tilyard Programme Development Team Noni Allison Rachael Clarke Rebecca Didham Terry Ehau Peter Ellison Dr Malcolm Kendall-Smith Dr Anne Marie Tangney Dr Trevor Walker Dr Sharyn Willis Dave Woods Report Development Team Justine Broadley Todd Gillies Lana Johnson Web Gordon Smith Design Michael Crawford Management and Administration Kaye Baldwin Tony Fraser Kyla Letman Professor Murray Tilyard Distribution Zane Lindon Lyn Thomlinson Colleen Witchall All information is intended for use by competent health care professionals and should be utilised in conjunction with © May 2008 pertinent clinical data. Contents Key points/purpose 2 Introduction 2 Background ▪ Haematopoiesis - Cell development 3 ▪ Limitations of reference ranges for the CBC 4 ▪ Borderline abnormal results must be interpreted in clinical context 4 ▪ History and clinical examination 4 White Cells ▪ Neutrophils 5 ▪ Lymphocytes 9 ▪ Monocytes 11 ▪ Basophils 12 ▪ Eosinophils 12 ▪ Platelets 13 Haemoglobin and red cell indices ▪ Low haemoglobin 15 ▪ Microcytic anaemia 15 ▪ Normocytic anaemia 16 ▪ Macrocytic anaemia 17 ▪ High haemoglobin 17 ▪ Other red cell indices 18 Summary Table 19 Glossary 20 This resource is a consensus document, developed with haematology and general practice input. We would like to thank: Dr Liam Fernyhough, Haematologist, Canterbury Health Laboratories Dr Chris Leathart, GP, Christchurch Dr Edward Theakston, Haematologist, Diagnostic Medlab Ltd We would like to acknowledge their advice, expertise and valuable feedback on this document. -

8 Erythrocyte Sedimentation Rate (Esr)
MODULE Erythrocyte Sedimentation Rate (ESR) Hematology and Blood Bank Technique 8 Notes ERYTHROCYTE SEDIMENTATION RATE (ESR) 8.1 INTRODUCTION Erythryocyte Sedimentation Rate (ESR) is the measurement after 1hour of the sedimentation of red cells when blood is allowed to stand in an open ended glass tube mounted vertically on a stand. OBJECTIVES After reading this lesson, you will be able to: z describe the methods used for measuring ESR z enlist the factors influencing the sedimentation of red cells z discuss the significance of measuring ESR z describe the reticulocyte count and it’s significance 8.2 METHODS OF MEASURING ESR ESR can be measured in the laboratory by two methods z Westergren method z Automated ESR analyser The International Council for Standardization in Hematology recommends the use of Westergren method as the standard method for measuring ESR. Westergren method The Westergren pipette is a glass pipette 30 cm in length and 2.5mm in diameter. The bore is uniform to within 5% throughout. A graduated scale in mm extends over the lower 20 cm. 62 HEMATOLOGY AND BLOOD BANK TECHNIQUE Erythrocyte Sedimentation Rate (ESR) MODULE Sample: Venous blood collected in EDTA. Four volumes of blood is diluted in Hematology and Blood 1 volume of citrate. Alternatively, 2ml blood is directly collected in 0.5ml of Bank Technique 3.8% trisodium citrate. Equipment required 1. Westergren pipette 2. Stainless steel rack for holding pipette Notes 3. Timer Method 1. Mix the blood sample well and draw in the Westergren pipette to the 0mm mark with a rubber teat. 2. Place the tube exactly vertical in the rack which has a rubber cork at the bottom. -

Successful Autologous Peripheral Blood Stem Cell Harvest and Transplantation After Splenectomy in a Patient with Multiple Myeloma with Hereditary Spherocytosis
International Journal of Myeloma 8(3): 11–15, 2018 CASE REPORT ©Japanese Society of Myeloma Successful autologous peripheral blood stem cell harvest and transplantation after splenectomy in a patient with multiple myeloma with hereditary spherocytosis Daisuke FURUYA1,4, Rikio SUZUKI1,4, Jun AMAKI1, Daisuke OGIYA1, Hiromichi MURAYAMA1,2, Hidetsugu KAWAI1, Akifumi ICHIKI1,3, Sawako SHIRAIWA1, Shohei KAWAKAMI1, Kaito HARADA1, Yoshiaki OGAWA1, Hiroshi KAWADA1 and Kiyoshi ANDO1 Hereditary spherocytosis (HS) is the most common inherited red cell membrane disorder worldwide. We herein report a 58-year-old male HS patient with mild splenomegaly who developed symptomatic multiple myeloma (MM). Autologous stem cell transplantation (ASCT) was considered to be adopted against MM, although there was a possibility of splenic rupture following stem cell mobilization. Therefore, splenectomy was performed prior to stem cell harvest, and he was able to safely mobilize sufficient CD34+ cells with G-CSF and plerixafor and undergo ASCT. This case suggests that stem cell mobilization after splenectomy is safe and effective in HS patients complicated with malignancies. Key words: multiple myeloma, hereditary spherocytosis, splenectomy, autologous peripheral blood stem cell harvest Introduction Consolidation with melphalan-based HDT followed by ASCT is still the standard treatment option for transplant-eligible Multiple myeloma (MM) is characterized by clonal prolifera- patients with MM, leading to higher complete response rates tion of abnormal plasma cells in the bone marrow (BM) micro- and increased progression-free survival and overall survival environment, monoclonal protein in the blood and/or urine, compared with conventional chemotherapy regimens [2]. bone lesions, and immunodeficiency [1]. In recent years, the Importantly, the emergence of novel agent-based therapy introduction of high-dose chemotherapy (HDT) and autol- combined with ASCT has revolutionized MM therapy [2]. -
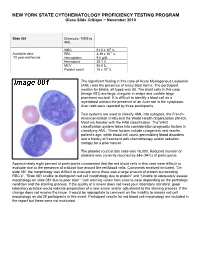
10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature
Henry Ford Hospital Medical Journal Volume 30 Number 4 Article 3 12-1982 Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature Michael R. Lovy Mark C. Kranc Gilbert B. Bluhm Jeanne M. Riddle Paul D. Stein Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Lovy, Michael R.; Kranc, Mark C.; Bluhm, Gilbert B.; Riddle, Jeanne M.; and Stein, Paul D. (1982) "Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature," Henry Ford Hospital Medical Journal : Vol. 30 : No. 4 , 189-198. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol30/iss4/3 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp Med J Vol 30, No 4,1982 Clinical Reports Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature Michael R. Lovy, MD,* Mark C. Krane, MD,** Gilbert B. Bluhm, MD, *** Jeanne M. Riddle, PhD,*** and Paul D. Stein, MD**** A hyperviscosity syndrome developed in two patients ity caused by the presence ofthe high molecular weight with rheumatoid arthritis. Analytic ultracentrifugation complexes. These abnormalities, as well as the clinical of the sera from one patient demonstrated 225 com bleeding, whole blood, and plasma viscosity, became plexes. Intermediate, 22S, and 375 complexes were normal after treatment. -

Red Blood Cell Aggregation in Preterm and Term Neonates and Adults
1356 LINDERKAMP ET AL. 003 1-3998/84/18 12-1356$02.00/0 PEDIATRIC RESEARCH Vol. 18, No. 12, 1984 Copyright O 1984 International Pediatric Research Foundation, Inc. Printed in U.S. A. Red Blood Cell Aggregation in Preterm and Term Neonates and Adults OTWIN LINDERKAMP, PATRICK OZANNE, PAUL Y. K. WU, AND HERBERT J. MEISELMAN Department of Pediatrics, University of Heidelberg, Federal Republic of Germany and Department of Physiology and Biophysics and Department of Pediatrics, University of Southern California, Los Angeles, California 90024 ABSTRACT. Aggregation of red blood cells (RBC) is a only during stasis or at low shear stresses (5, 22, 23). ~t high major determinant of blood viscosity and of blood circula- shear stresses (about 3 dynes/cm2 or more), RBC aggregates are tion through vessels with slow flow (i.e. veins). RBC ag- rapidly dispersed (23). Thus, RBC aggregation usually takes place gregation and plasma fibrinogen were studied in placental only in the venous portion of the circulation where the blood blood samples from 25 neonates with 24 to 41 wk of flow rate is slow and the fluid shear stresses are low. RBC gestation and in blood from 13 normal adults. The rate and aggregation may occur in other sections of the circulation, how- final extent of RBC aggregation were measured by means ever, under pathophysiological situations of reduced blood flow of a rheoscope (increase in light transmission during blood (i.e. shock). Conversely, increased RBC aggregation (e.g. as a stasis). Both the rate and extent of RBC aggregation were result of high fibrinogen level) can cause lower blood flow rates, low in the premature infants, increased with gestational and this latter mechanism has been suggested as a possible factor age, and reached the highest values in the adults. -
Blood Lab First Week
Blood Lab First Week This is a self propelled powerpoint study atlas of blood cells encountered in examination of the peripheral blood smear. It is a requirement of this course that you begin review of these slides with the first day of class in order to familiarize yourself with terms used in describing peripheral blood morphology. Laboratory final exam questions are derived directly from these slides. The content of these slides may duplicate slides (1-37) listed on the Hematopathology Website. You must familiarize yourself with both sets of slides in order to have the best learning opportunity. Before Beginning This Slide Review • Please read the Laboratory information PDF under Laboratory Resources file to learn about – Preparation of a blood smear – How to select an area of the blood smear for review of morphology and how to perform a white blood cell differential – Platelet estimation – RBC morphology descriptors • Anisocytosis • Poikilocytosis • Hypochromia • Polychromatophilia Normal blood smear. Red blood cells display normal orange pink cytoplasm with central pallor 1/3-1/2 the diameter of the red cell. There is mild variation in size (anisocytosis) but no real variation in shape (poikilocytosis). To the left is a lymphocyte. To the right is a typical neutrophil with the usual 3 segmentations of the nucleus. Med. Utah pathology Normal blood: thin area Ref 2 Normal peripheral blood smear. This field is good for exam of cell morphology, although there are a few minor areas of overlap of red cells. Note that most cells are well dispersed and the normal red blood cell central pallor is noted throughout the smear. -

Life Is in the Blood
Scholars Crossing Faculty Publications and Presentations Department of Biology and Chemistry 8-2-2019 Life Is in the Blood Alan L. Gillen Liberty University, [email protected] Jason Conrad Follow this and additional works at: https://digitalcommons.liberty.edu/bio_chem_fac_pubs Part of the Biology Commons Recommended Citation Gillen, Alan L. and Conrad, Jason, "Life Is in the Blood" (2019). Faculty Publications and Presentations. 148. https://digitalcommons.liberty.edu/bio_chem_fac_pubs/148 This Article is brought to you for free and open access by the Department of Biology and Chemistry at Scholars Crossing. It has been accepted for inclusion in Faculty Publications and Presentations by an authorized administrator of Scholars Crossing. For more information, please contact [email protected]. Life Is in the Blood by Dr. Alan L. Gillen and Jason Conrad on August 2, 2019 Keywords: Blood, Circulation, Leeuwenhoek, red blood cells, white blood cells, capillary, life is in the blood, erythrocyte, leukocyte, rouleaux Abstract It takes about 60 seconds for all the blood in your body to complete its journey. It travels from your heart to your extremities and returns, there and back again. Blood moves with the rapid current of the great arterial rivers and through the smallest capillary creeks. William Harvey first noticed circulation (1628) through the heart into arteries and veins; however, he could not see how they connected since he did not have a microscope. The man who first described this was Anton van Leeuwenhoek about 46 years later (1674). Then, J. J. Lister and Thomas Hodgkin described the rouleaux formation or stacking of RBCs through a capillary bed. -

BLOOD CELL IDENTIFICATION Educational Commentary Is
EDUCATIONAL COMMENTARY – BLOOD CELL IDENTIFICATION Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on the left side of the screen. Learning Objectives: After completion of this testing event, the participant will be able to: • Describe morphologic features of normal peripheral blood leukocytes and platelets. • Identify morphologic abnormalities in erythrocytes and leukocytes associated with Waldenström's macroglobulinemia. The patient presented in the case study for this testing event has been diagnosed with Waldenström's macroglobulinemia. The images for review represent not only normal leukocytes and platelets, but also several abnormalities in red blood cells that may be seen in this condition. The photographs also include a leukocyte (plasma cell) that is not normally viewed in the peripheral blood. BCI-15 depicts a band (stab) neutrophil. Band cells are the earliest precursors of neutrophil maturation that can be normally visualized in the peripheral blood. They characteristically have a nucleus that is shaped like the letters “C” or “U”. The chromatin is clumped and dense. The cytoplasm in band cells contains many specific granules that stain tan, pink, or violet. Picture BCI-16 shows a polychromatophilic erythrocyte. This cell actually represents the stage of red blood cell maturation just prior to the mature erythrocyte, the reticulocyte. The reticulocyte stage begins just after the nucleus has been extruded by the erythrocyte. A small amount of RNA (ribonucleic acid) is still present in the cells and therefore the cell appears blue-gray when Wright’s stain is used. -

Rouleaux Formation of White Blood Cells and Platelets in Leukemia
410 Original Article ROULEAUX FORMATION OF WHITE BLOOD CELLS AND PLATELETS IN LEUKEMIA FORMAÇÃO DE ROULEAUX DE GLÓBULOS BRANCOS E PLAQUETAS EM LEUCEMIA 1 2 2 2 3 Hafeez ULLAH ; Khalid NAEEM ; Munir AKHTAR ; Fayyaz HUSSAIN ; Mukhtar AHMAD 1. Biohotonics Research laboratory, Department of Physics, The Islamia University of Bahawalpur, Bahawalpur, Pakistan; 2. Laser and Optronics Laboratory, Department of Physics, Bahauddin Zakariya University, Unive Bosan Road, Multan, Pakistan; 3. Department of Physics, COMSATS Institute of Information Technology, Lahore, Pakistan. ABSTRACT: The objective of this study was to measure the effects of glucose and salt level on white blood cells, red blood cells and platelets (PLTs) in the blood of a leukemic patient by using a white light microscope. Different concentrations of glucose and salt in the range of 0 mM to 500 mM were admixed in the blood sample to prepare blood smear. We revealed that shape of erythrocytes, leukocytes and platelets changes and form aggregates. Increasing concentrations of glucose cause to increases aggregation process of white blood cells, red blood cells and platelets. And the increasing concentration of sodium chloride causes to increase rouleaux formation and aggregation of platelets but dehydration due to increased sodium chloride concentration causes to break the aggregation of white blood cells. Comparison of CBC reports of these samples with and without analytes shows that total leukocyte count (TLC) decreases gradually towards normal ranges of leukocytes which is favorable in the treatment of leukemia but at the same time decreasing level of hemoglobin HGB, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and increasing level of red blood cell (RBCs) causes to reduce oxygen supply which is in favor of cancer growth and anemia.