Extraction of Vanadium from an Industrial Waste
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vanadium Pentoxide and Other Inorganic Vanadium Compounds
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 29 VANADIUM PENTOXIDE AND OTHER INORGANIC VANADIUM COMPOUNDS Note that the layout and pagination of this pdf file are not identical to the printed CICAD First draft prepared by Dr M. Costigan and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2001 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -
US Schedule for Internet V2
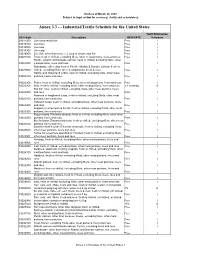
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Study on New Desilication Technology of High Silica Bauxite
2016 2nd International Conference on Sustainable Energy and Environmental Engineering (SEEE 2016) ISBN: 978-1-60595-408-0 Study on New Desilication Technology of High Silica Bauxite Hai-yun XIE 1, Chao DING 1, Peng-fei ZHANG 1,2 , Lu-zheng CHEN 1, and Xiong-TONG 1 1Faculty of Land Resource Engineering, Kunming University of Science and Technology, Kunming 650093, China 2State Key Laboratory of Complex Nonferrous Metal Resource Clean Utilization, Kunming 650093, China Keywords: High silicon bauxite, Desilication, Gravity separation, Froth flotation. Abstract: There are abundant diasporic bauxite resource in China. The gravity separation and flotation process are utilized for treating this kind of ore. When the ore grinding fineness is -200 mesh 80%, the qualified bauxite concentrate can be obtained, and it contains Al 2O3 71.62%, SiO 2 8.94%, and its Al 2O3 recovery rate is 75.43%, A/S is 8.02.Compared with single flotation process, it can be reduced for the dosage flotation reagent and the cost of mineral processing. Introduction China's bauxite resources are rich, the amount of resources of 1.914 billion, ranking the fifth in the world, but more than 80% are medium and low grade ore, Aluminum silicon ratio (A/S) between 4 to 7 of the bauxite accounted for 59.5%. These minerals can not meet the basic conditions for the production of alumina by Bayer process. (A/S>8) [1]. The vast majority of China's bauxite belong to high-alumina, high-silicon, fine-grained embedded diaspore-type bauxite, so the bauxite is difficult to concentrate. -

Ferro Vanadium Production Business. Ferrovanadium Market Is Expected to Expand at a CAGR of 5.0% Between 2018 and 2028
www.entrepreneurindia.co Introduction Ferro Vanadium is an alloy which is formed by combining iron and vanadium with a vanadium content range of 35%-85%. Ferro Vanadium is a universal hardener, strengthener and anti-corrosive additive for steels like high-strength low-alloy (HSLA) steel, tool steels, as well as other ferrous-based products. Ferro vanadium belongs to the category of ferroalloy. Ferro vanadium is an alloy which is formed by combining iron and vanadium. Ferrovanadium contains 35% to 85% of vanadium depending on applications of the product in end-use industry. Ferro vanadium is an alloy material that is used in manufacturing of steel. It imparts desirable properties such as abrasion resistance, high temperature and hardenability. www.entrepreneurindia.co Ferro vanadium used for manufacturing of steel offers the end product with high stability against alkalis as well as acids such as sulphuric and hydrochloric acids. In addition, products containing ferro vanadium are at reduced risk to be susceptible to corrosion. Ferro vanadium also helps in reducing the overall weight of the material as well as increasing the overall tensile strength of the end product. In addition, it helps in promoting fine grain size and increasing hardenability through precipitation of nitrides and carbides. Ferro vanadium is manufactured using an electric arc furnace in which scrap iron is melted initially and then it is combined with the mixture of aluminum as well as flux such as calcium fluoride and calcium oxide. It is usually supplied in pallet boxes or in shrink wrapped in super bags. www.entrepreneurindia.co Ferro Vanadium is produced from Vanadium Sludge & usually available in the range with V: 50-85%. -

Iron Recovery from Bauxite Tailings Red Mud by Thermal Reduction with Blast Furnace Sludge
applied sciences Article Iron Recovery from Bauxite Tailings Red Mud by Thermal Reduction with Blast Furnace Sludge Davide Mombelli * , Silvia Barella , Andrea Gruttadauria and Carlo Mapelli Dipartimento di Meccanica, Politecnico di Milano, via La Masa 1, Milano 20156, Italy; [email protected] (S.B.); [email protected] (A.G.); [email protected] (C.M.) * Correspondence: [email protected]; Tel.: +39-02-2399-8660 Received: 26 September 2019; Accepted: 9 November 2019; Published: 15 November 2019 Abstract: More than 100 million tons of red mud were produced annually in the world over the short time range from 2011 to 2018. Red mud represents one of the metallurgical by-products more difficult to dispose of due to the high alkalinity (pH 10–13) and storage techniques issues. Up to now, economically viable commercial processes for the recovery and the reuse of these waste were not available. Due to the high content of iron oxide (30–60% wt.) red mud ranks as a potential raw material for the production of iron through a direct route. In this work, a novel process at the laboratory scale to produce iron sponge ( 1300 C) or cast iron (> 1300 C) using blast furnace ≤ ◦ ◦ sludge as a reducing agent is presented. Red mud-reducing agent mixes were reduced in a muffle furnace at 1200, 1300, and 1500 ◦C for 15 min. Pure graphite and blast furnace sludges were used as reducing agents with different equivalent carbon concentrations. The results confirmed the blast furnace sludge as a suitable reducing agent to recover the iron fraction contained in the red mud. -

PRODUCTION and REFINING of METALS (Electrolytic C25); PRETREATMENT of RAW MATERIALS
C22B PRODUCTION AND REFINING OF METALS (electrolytic C25); PRETREATMENT OF RAW MATERIALS Definition statement This subclass/group covers: Metallurgical or chemical processes for producing or recovering metals from metal compounds, ores, waste or scrap metal and for refining metal. Included in this subclass are processes drawn to: the production of metal by smelting, roasting or furnace method; the extraction of metal compounds from ore and concentrates by wet processes; electrochemical treatment of ores and metallurgical products for obtaining metals or alloys; apparatus thereof; preliminary treatment of ores, concentrates and scrap; general process for refining or remelting metals; apparatus for electroslag or arc remelting of metals; obtaining specific metals; consolidating metalliferous charges or treating agents that are subsequently used in other processes of this subclass, by agglomerating, compacting, indurating or sintering. Relationship between large subject matter areas This subclass covers the treatment, e.g. decarburization, of metallferrous material for purposes of refining. C21C, C21D and C22F provide decarburization of metal for modifying the physical structure of ferrous and nonferrous metals or alloys, respectively. C22B also possesses groups for obtaining metals including obtaining metals by chemical processes, and obtaining metal compounds by metallurgical processes. Thus, for example, group C22B 11/00 covers the production of silver by reduction of ammoniacal silver oxide in solution, and group C22B 25/00 covers the -

Iron-Rich Bauxite and Bayer Red Muds Yingyi Zhang, Yuanhong Qi and Jiaxin Li
Chapter Aluminum Mineral Processing and Metallurgy: Iron-Rich Bauxite and Bayer Red Muds Yingyi Zhang, Yuanhong Qi and Jiaxin Li Abstract Bauxite is the main source for alumina production. With the rapid development of iron and steel industry and aluminum industry, high-quality iron ore and bauxite resources become increasingly tense. However, a lot of iron-rich bauxite and Bayer red mud resources have not been timely and effectively recycled, resulting in serious problems of environmental pollution and wastage of resources. The com- prehensive utilization of iron-rich bauxite and red mud is still a worldwide problem. The industrial stockpiling is not a fundamental way to solve the problems of iron- rich bauxite and red mud. As to the recovery of valuable metals from iron-rich bauxite and red mud, there are a lot of technical and cost problems, which are serious impediments to industrial development. Applying red mud as construction materials like cement, soil ameliorant applications face the problem of Na, Cr, As leaching into the environment. However, the high-temperature reduction, smelting and alkaline leaching process is a feasible method to recover iron and alumina from iron-rich bauxite and red mud. This chapter intends to provide the reader an overview on comprehensive utilization technology of the low-grade iron-rich bauxite and Bayer red mud sources. Keywords: bauxite, iron-rich bauxite, Bayer red mud, Bayer process, magnetic separation, reduction and smelting process 1. Introduction With 8.30%, aluminum is the third element in the earth’s crust after the oxygen and the silicon, and for that reason, aluminum minerals are abundant with more than 250 kinds. -

The Determination of Vanadium and Chromium in Ferrovanadium by Electrometric Titration' by G
Oct., 1921 THE JOURNAL OF INDUSTRIAL AND ENGINEERING CHEMISTRY 939 CONCLUSIONS Modifications in bleaching methods give promise of greater The chemical characteristics of soda and sulfate pulps result,s than modifying cooking methods. indicate that they are a very pure form of wood cellulose and Modifications in which the bleaching operation was divided capable of high yields of white fibrous and resistant material. into two steps, with washing between steps, cut the bleach The sulfate process is much more efficient than the soda requirement in two. process in yielding a bleachable pulp from coniferous wood. Pulps of better quality, both from physical and chemical The coloring matter in pulps is of the nature of a dye and considerations, are obtained by cooking the wood as little can be removed without materially reducing yields. Most as possible in isolating the fibers and by accomplishing as of the action in cooking to reduce bleach consumption is to much of the burden of purification as possible in the bleaching dissolve and degrade the cellulose. and washing operations. The Determination of Vanadium and Chromium in Ferrovanadium by Electrometric Titration' By G. L. Kelley, J. A. Wiley, R. T. Bohn and W. e. Wright MIDVALESTEEL & ORDNANCE C0.p NICETOWNPLANT, PHILADELPHIA, PENNSYLVANtd The object of this paper is to describe a method for the When the vanadyl sulfate is titrated at 80" C. with perman- determination of vanadium in ferrovanadium not subject ganate in a similar acid concentration, the change of poten- to interference by chromium, which is so often present. Kel- tial is about 60 mv. -

Extraction of Aluminium from Coal Fly Ash Using a Two-Step Acid Leach Process
EXTRACTION OF ALUMINIUM FROM COAL FLY ASH USING A TWO-STEP ACID LEACH PROCESS Alan Shemi A dissertation submitted to the faculty of Engineering and the Built Environment, University of Witwatersrand, Johannesburg, in fulfillment of the requirements for the degree of Master of Science in Engineering Extraction of Aluminium from CFA Alan Shemi DECLARATION I declare that this dissertation is my own unaided work. It is being submitted to the degree of Master of Science in Engineering to the University of the Witwatersrand, Johannesburg. It has not been submitted before for any other degree or examination in any other University. ---------------------------------- Alan Shemi 14th Day of May 2013 Page ii Extraction of Aluminium from CFA Alan Shemi ABSTRACT Hydrometallurgical extraction technologies provide a process route for resource recovery of valuable metals from both primary as well as secondary resources. In this study, the possibility of treating coal fly ash (CFA), a residue formed as a result of coal combustion in coal-fired power plants, was investigated. Eskom CFA contains significant amounts of alumina typically, 26-31%, in two dissimilar phases, namely amorphous and crystalline mullite, which may be processed separately. Due to its high silica content, however, CFA cannot be treated through the Bayer process route. Therefore, a leach-sinter-leach process was formulated that employed a two-step acid leach technique to extract alumina from CFA using sulphuric acid. In the preliminary test work, the effect of parameters on CFA leaching characteristics was investigated. From the experimental results, appropriate factor levels were found to be 6M acid concentration, 6 hours leaching time, 75°C temperature and 1:4 solid to liquid ratio. -

Recovery of Desilication Product in Alumina Industry
Recovery of Desilication Product in Alumina Industry Hasitha Indrajith de Silva B.Sc. Engineering (Honours) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2013 School of Chemical Engineering Abstract Bayer process is used for the production of alumina from bauxite which contains siliceous minerals known as reactive silica. Reactive silica is also digested during the Bayer process, forming desilication product (DSP) which traps significant amount of sodium from the caustic soda used. DSP containing red mud is discarded to the bauxite residue storage areas causing alarming economic and environmental concerns to the alumina industry. Separation of DSP from red mud is a critical step to subsequent sodium recovery. Therefore, methods of separating DSP from red mud originated in a Western Australian alumina refinery were investigated in the present study. Red mud classified into five size classes was characterised using particle size measurement, material density, X-ray diffraction, X-ray photon spectroscopy, electron microscopy fitted with energy dispersive spectroscopy. Size separation alone resulted in an increase of DSP from ~7% to 12-14% in finer size classes of red mud. Since red mud contains more than 50% iron oxides, dissolution of these oxides would invariably result in an increase of DSP, therefore, several dissolution mechanisms of these oxides were investigated. Sodium Citrate-Bicarbonate-Dithionite (CBD) method resulted in an increase of DSP content from ~12 to 22%, while a comparative study was also carried out using deferroxiamine B (DFO-B) as the complexing agent. The use of ultrasonically assisted-CBD resulted in a significant improvement of iron dissolution from red mud. -

CHANGING TECHNOLOGY Ln a CHANGING WORLD the CASE of EXTRACTIVE METALLURGY 1
CHANGING TECHNOLOGY lN A CHANGING WORLD THE CASE OF EXTRACTIVE METALLURGY 1 Fathi Habashi 2 ABSTRACT Smelting of ores for metal extraction is an ancient art in our civilization. New technology is being introduced, but forces of resistance are sometimes so strong to permit a radical change. While the iron and steel, zinc, aluminum, and gold industries are responding to change, the copper and nickel industries seem to be more conservative. Key words: iron, steel, copper, pressure hydrometallurgy, gold, alurninum, zinc, nickel, sulfur dioxide, elemental sulfur, sulfuric acid, pyrite, arsenopyrite. ' XVII Encontro Nacional de Tratamento de Minérios e Metalurgia Extrativa - I Simpósio sobre Química de Colóides- Aguas de Sao Pedro- SP, Brazil , August 23-26. 1998. :· Department of Mining and Metallurgy, Lavai University, Qut:bec City, Canada 49 INTRODUCTION Extractive metallurgy today is usually divided in two sectors: ferrous and nonferrous because of the large scale operations in the ferrous [1). Steel production in one year exceeds that of ali other metais combined in ten years. As a result, metallurgists in the nonferrous sector usually do not participate in the activities of iron and steel makers and vice versa. This is a pity because for sure one can leam from the other. ln fact, the copper industry adopted many technologies used first in iron and steel production. For example, when Bessemer invented the conventer in 1856 it was adopted in the copper industry ten years !ater, and when high grade massive copper sulfide ores were exhausted and metallurgists were obliged to treat low-grade ores, the puddling furnace was adapted in form of a reverberatory furnace to treat the flotation concentrates. -

Toxicological Profile for Vanadium
TOXICOLOGICAL PROFILE FOR VANADIUM U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry September 2012 VANADIUM ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. VANADIUM iii UPDATE STATEMENT A Toxicological Profile for Vanadium, Draft for Public Comment was released in September 2009. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Human Health Sciences (proposed) Environmental Toxicology Branch (proposed) 1600 Clifton Road NE Mailstop F-62 Atlanta, Georgia 30333 VANADIUM iv This page is intentionally blank. VANADIUM v FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the toxic substances each profile describes. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced.