Hesperidin As a Neuroprotective Agent: a Review of Animal and Clinical Evidence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thesis of Potentially Sweet Dihydrochalcone Glycosides
University of Bath PHD The synthesis of potentially sweet dihydrochalcone glycosides. Noble, Christopher Michael Award date: 1974 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 05. Oct. 2021 THE SYNTHESIS OF POTBTTIALLY SWEET DIHYDROCHALCOITB GLYCOSIDES submitted by CHRISTOPHER MICHAEL NOBLE for the degree of Doctor of Philosophy of the University of Bath. 1974 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author.This copy of the the sis has been supplied on condition that anyone who con sults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be pub lished without the prior written consent of the author. -

Plant Phenolics: Bioavailability As a Key Determinant of Their Potential Health-Promoting Applications
antioxidants Review Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications Patricia Cosme , Ana B. Rodríguez, Javier Espino * and María Garrido * Neuroimmunophysiology and Chrononutrition Research Group, Department of Physiology, Faculty of Science, University of Extremadura, 06006 Badajoz, Spain; [email protected] (P.C.); [email protected] (A.B.R.) * Correspondence: [email protected] (J.E.); [email protected] (M.G.); Tel.: +34-92-428-9796 (J.E. & M.G.) Received: 22 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids. Interest in phenolic compounds has dramatically increased during the last decade due to their biological effects and promising therapeutic applications. In this review, we discuss the importance of phenolic compounds’ bioavailability to accomplish their physiological functions, and highlight main factors affecting such parameter throughout metabolism of phenolics, from absorption to excretion. Besides, we give an updated overview of the health benefits of phenolic compounds, which are mainly linked to both their direct (e.g., free-radical scavenging ability) and indirect (e.g., by stimulating activity of antioxidant enzymes) antioxidant properties. Such antioxidant actions reportedly help them to prevent chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases, among others. Last, we comment on development of cutting-edge delivery systems intended to improve bioavailability and enhance stability of phenolic compounds in the human body. Keywords: antioxidant activity; bioavailability; flavonoids; health benefits; phenolic compounds 1. Introduction Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1]. -

Novel Neohesperidin Dihydrochalcone Analogue Inhibits Adipogenic Differentiation of Human Adipose-Derived Stem Cells Through the Nrf2 Pathway
International Journal of Molecular Sciences Article Novel Neohesperidin Dihydrochalcone Analogue Inhibits Adipogenic Differentiation of Human Adipose-Derived Stem Cells through the Nrf2 Pathway Ga Eun Han 1,†, Hee-Taik Kang 2,† ID , Sungkyun Chung 3, Changjin Lim 3, John A. Linton 4, Jin-Hee Lee 1, Wooki Kim 5, Seok-Ho Kim 3,* and Jong Hun Lee 1,* 1 Department of Food science and Biotechnology, College of Life Science, CHA University, Seongnam-si 13488, Korea; [email protected] (G.E.H.); [email protected] (J.-H.L.) 2 Department of Family Medicine, College of Medicine, Chungbuk National University, Chungbuk 28644, Korea; [email protected] 3 Department of Pharmacy, College of Pharmacy and Institute of Pharmaceutical Sciences, CHA University, Seongnam-si 13488, Korea; [email protected] (S.C.); [email protected] (C.L.) 4 Department of Family Medicine, Severance Hospital, Yonsei University, Seoul 03722, Korea; [email protected] 5 Department of Food Science and Biotechnology, Graduate School of Biotechnology, Kyung Hee University, Yongin 17104, Korea; [email protected] * Correspondence: [email protected] (S.-H.K.); [email protected] (J.H.L.); Tel.: +82-31-881-7169 (S.-H.K.); +82-31-881-7158 (J.H.L.) † These authors contributed equally to this work. Received: 18 June 2018; Accepted: 24 July 2018; Published: 29 July 2018 Abstract: Obesity, characterized by excess lipid accumulation, has emerged as a leading public health problem. Excessive, adipocyte-induced lipid accumulation raises the risk of metabolic disorders. Adipose-derived stem cells (ASCs) are mesenchymal stem cells (MSCs) that can be obtained from abundant adipose tissue. -

Supplementary Materials Evodiamine Inhibits Both Stem Cell and Non-Stem
Supplementary materials Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70 Seung Yeob Hyun, Huong Thuy Le, Hye-Young Min, Honglan Pei, Yijae Lim, Injae Song, Yen T. K. Nguyen, Suckchang Hong, Byung Woo Han, Ho-Young Lee - 1 - Table S1. Short tandem repeat (STR) DNA profiles for human cancer cell lines used in this study. MDA-MB-231 Marker H1299 H460 A549 HCT116 (MDA231) Amelogenin XX XY XY XX XX D8S1179 10, 13 12 13, 14 10, 14, 15 13 D21S11 32.2 30 29 29, 30 30, 33.2 D7S820 10 9, 12 8, 11 11, 12 8 CSF1PO 12 11, 12 10, 12 7, 10 12, 13 D3S1358 17 15, 18 16 12, 16, 17 16 TH01 6, 9.3 9.3 8, 9.3 8, 9 7, 9.3 D13S317 12 13 11 10, 12 13 D16S539 12, 13 9 11, 12 11, 13 12 D2S1338 23, 24 17, 25 24 16 21 D19S433 14 14 13 11, 12 11, 14 vWA 16, 18 17 14 17, 22 15 TPOX 8 8 8, 11 8, 9 8, 9 D18S51 16 13, 15 14, 17 15, 17 11, 16 D5S818 11 9, 10 11 10, 11 12 FGA 20 21, 23 23 18, 23 22, 23 - 2 - Table S2. Antibodies used in this study. Catalogue Target Vendor Clone Dilution ratio Application1) Number 1:1000 (WB) ADI-SPA- 1:50 (IHC) HSP70 Enzo C92F3A-5 WB, IHC, IF, IP 810-F 1:50 (IF) 1 :1000 (IP) ADI-SPA- HSP90 Enzo 9D2 1:1000 WB 840-F 1:1000 (WB) Oct4 Abcam ab19857 WB, IF 1:100 (IF) Nanog Cell Signaling 4903S D73G4 1:1000 WB Sox2 Abcam ab97959 1:1000 WB ADI-SRA- Hop Enzo DS14F5 1:1000 WB 1500-F HIF-1α BD 610958 54/HIF-1α 1:1000 WB pAkt (S473) Cell Signaling 4060S D9E 1:1000 WB Akt Cell Signaling 9272S 1:1000 WB pMEK Cell Signaling 9121S 1:1000 WB (S217/221) MEK Cell Signaling 9122S 1:1000 -

Flavonoid Glucodiversification with Engineered Sucrose-Active Enzymes Yannick Malbert
Flavonoid glucodiversification with engineered sucrose-active enzymes Yannick Malbert To cite this version: Yannick Malbert. Flavonoid glucodiversification with engineered sucrose-active enzymes. Biotechnol- ogy. INSA de Toulouse, 2014. English. NNT : 2014ISAT0038. tel-01219406 HAL Id: tel-01219406 https://tel.archives-ouvertes.fr/tel-01219406 Submitted on 22 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Last name: MALBERT First name: Yannick Title: Flavonoid glucodiversification with engineered sucrose-active enzymes Speciality: Ecological, Veterinary, Agronomic Sciences and Bioengineering, Field: Enzymatic and microbial engineering. Year: 2014 Number of pages: 257 Flavonoid glycosides are natural plant secondary metabolites exhibiting many physicochemical and biological properties. Glycosylation usually improves flavonoid solubility but access to flavonoid glycosides is limited by their low production levels in plants. In this thesis work, the focus was placed on the development of new glucodiversification routes of natural flavonoids by taking advantage of protein engineering. Two biochemically and structurally characterized recombinant transglucosylases, the amylosucrase from Neisseria polysaccharea and the α-(1→2) branching sucrase, a truncated form of the dextransucrase from L. Mesenteroides NRRL B-1299, were selected to attempt glucosylation of different flavonoids, synthesize new α-glucoside derivatives with original patterns of glucosylation and hopefully improved their water-solubility. -

Glycosides in Lemon Fruit
Food Sci. Technol. Int. Tokyo, 4 (1), 48-53, 1998 Characteristics of Antioxidative Flavonoid Glycosides in Lemon Fruit Yoshiaki MIYAKE,1 Kanefumi YAMAMOT0,1 Yasujiro MORIMITSU2 and Toshihiko OSAWA2 * Central Research Laboratory of Pokka Corporation, Ltd., 45-2 Kumanosyo, Shikatsu-cho, Nishikasugai-gun, Aichi 481, Japan 2Department of Applied Biological Sciences, Nagoya University, Nagoya 46401, Japan Received June 12, 1997; Accepted September 27, 1997 We investigated the antioxidative flavonoid glycosides in the peel extract of lemon fruit (Citrus limon). Six flavanon glycosides: eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, and neohesperidin, and three flavone glycosides: diosmin, 6~-di- C-p-glucosyldiosmin (DGD), and 6- C-p-glucosyldiosmin (GD) were identified by high- performance liquid chromatography (HPLC) analysis. Their antioxidative activity was examined using a linoleic acid autoxidation system. The antioxidative activity of eriocitrin, neoeriocitrin and DGD was stronger than that of the others. Flavonoid glycosides were present primarily in the peel of lemon fruit. There was only a small difference in the content of the flavonoid glycosides of the lemon fruit juice from various sources and varieties. Lemon fruit contained abundant amounts of eriocitrin and hesperidin and also contained narirutin, diosmin, and DGD, but GD, neoeriocitrin, naringin, and neohesperidin were present only in trace amounts. The content of DGD, GD, and eriocitrin was especially abundant in lemons and limes; however, they were scarcely found in other citrus fruits. The content of flavonoid compounds in lemon juice obtained by an in-line extractor at a juice factory was more abundant than that obtained by hand-squeezing. These compounds were found to be stable even under heat treatment conditions (121'C, 15 min) in acidic solution. -

GRAS Notice (GRN) No.901, Glucosyl Hesperidin
GRAS Notice (GRN) No. 901 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory ~~~lECTV!~ITJ) DEC 1 2 20,9 OFFICE OF FOOD ADDITI\/c SAFETY tnC Vanguard Regulator~ Services, Inc 1311 Iris Circle Broomfield, CO, 80020, USA Office: + 1-303--464-8636 Mobile: +1-720-989-4590 Email: [email protected] December 15, 2019 Dennis M. Keefe, PhD, Director, Office of Food Additive Safety HFS-200 Food and Drug Administration 5100 Paint Branch Pkwy College Park, MD 20740-3835 Re: GRAS Notice for Glucosyl Hesperidin Dear Dr. Keefe: The attached GRAS Notification is submitted on behalf of the Notifier, Hayashibara Co., ltd. of Okayama, Japan, for Glucosyl Hesperidin (GH). GH is a hesperidin molecule modified by enzymatic addition of a glucose molecule. It is intended for use as a general food ingredient, in food. The document provides a review of the information related to the intended uses, manufacturing and safety of GH. Hayashibara Co., ltd. (Hayashibara) has concluded that GH is generally recognized as safe (GRAS) based on scientific procedures under 21 CFR 170.30(b) and conforms to the proposed rule published in the Federal Register at Vol. 62, No. 74 on April 17, 1997. The publically available data and information upon which a conclusion of GRAS was made has been evaluated by a panel of experts who are qualified by scientific training and experience to assess the safety of GH under the conditions of its intended use in food. A copy of the Expert Panel's letter is attached to this GRAS Notice. -

Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix
antioxidants Article Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion Marcella Denaro †, Antonella Smeriglio *,† and Domenico Trombetta Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Viale Palatucci, 98168 Messina, Italy; [email protected] (M.D.); [email protected] (D.T.) * Correspondence: [email protected]; Tel.: +39-090-676-4039 † Both authors contributed equally. Abstract: Recently, several studies have highlighted the role of Citrus flavanones in counteracting oxidative stress and inflammatory response in bowel diseases. The aim of study was to identify the most promising Citrus flavanones by a preliminary antioxidant and anti-inflammatory screening by in vitro cell-free assays, and then to mix the most powerful ones in equimolar ratio in order to investigate a potential synergistic activity. The obtained flavanones mix (FM) was then subjected to in vitro simulated digestion to evaluate the availability of the parent compounds at the intestinal level. Finally, the anti-inflammatory activity was investigated on a Caco-2 cell-based model stimulated with interleukin (IL)-1β. FM showed stronger antioxidant and anti-inflammatory activity with respect to the single flavanones, demonstrating the occurrence of synergistic activity. The LC-DAD-ESI- MS/MS analysis of gastric and duodenal digested FM (DFM) showed that all compounds remained unchanged at the end of digestion. As proof, a superimposable behavior was observed between FM and DFM in the anti-inflammatory assay carried out on Caco-2 cells. Indeed, it was observed that both FM and DFM decreased the IL-6, IL-8, and nitric oxide (NO) release similarly to the reference Citation: Denaro, M.; Smeriglio, A.; anti-inflammatory drug dexamethasone. -

8 Weeks of 2S-Hesperidin Supplementation Improves Power Output at Estimated Functional Threshold Power and Maximum Power in Amat
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 16 September 2020 doi:10.20944/preprints202009.0341.v1 1 Article 2 8 Weeks of 2s-Hesperidin Supplementation Improves Power Output 3 at Estimated Functional Threshold Power and Maximum Power in 4 Amateur Cyclist 5 Full names of the authors and institutional/corporate affiliations 6 Francisco Javier Martínez Noguera *1, Cristian Marín Pagán 1, Jorge Carlos Vivas 7 2, Pedro E. Alcaraz 1. 8 1 Research Center for High Performance Sport. Catholic University of Murcia, campus de 9 los Jerónimos Nº 135 (UCAM, 30107, Murcia, Spain). 10 2 Health, Economy, Motricity and Education Research Group (HEME), Faculty of Sport 11 Sciences, University of Extremadura. Avda. de Elvas, s/n. (06006, Badajoz, Spain), 12 13 Contact details for the corresponding author 14 * Francisco Javier Martínez Noguera: Research Center for High Performance Sport. 15 Catholic University of Murcia, campus de los Jerónimos Nº 135 (UCAM, 30107, Murcia, 16 Spain); [email protected].; Tel.: +34 968 278 566 (F.J.M.N.) 17 18 Email addresses 19 FJMN: [email protected]. 20 CMP: [email protected]. 21 JCV: [email protected]. 22 PEA: [email protected]. 23 24 25 26 27 28 © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 16 September 2020 doi:10.20944/preprints202009.0341.v1 29 ABSTRACT 30 2S-hesperidin is a flavanone (flavonoid) found in high concentrations in citrus 31 fruits. It has an antioxidant and anti-inflammatory effect, improving performance 32 in animals. This study investigated the effects of chronic intake of an orange extract 33 (2S-hesperidin) or placebo on aerobic-anaerobic and metabolic performance 34 markers in amateur cyclists. -
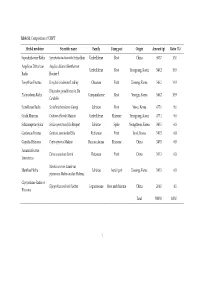
Ratio (%) Saposhnikoviae Ra
Table S1. Composition of CSBPT Herbal medicine Scientific name Family Using part Origin Amount (g) Ratio (%) Saposhnikoviae Radix Saposhnikovia divaricate Schischkin Umbelliferae Root China 500.7 10.0 Angelicae Dahuricae Angelica dahurica Bentham et Umbelliferae Root Yeongyang, Korea 546.2 10.9 Radix Hooker F. Forsythiae Fructus Forsythia viridissima Lindley Oleaceae Fruit Uiseong, Korea 546.2 10.9 Platycodon grandiflorum A. De Platycodonis Radix Campanulaceae Root Yeongju, Korea 546.2 10.9 Candolle Scutellariae Radix Scutellaria baicalensis Georgi Labiatae Root Yeosu, Korea 477.1 9.6 Cnidii Rhizoma Cnidium officinale Makino Umbelliferae Rhizome Yeongyang, Korea 477.1 9.6 Schizonepetae Spica Schizonepeta tenuifolia Briquet Labiatae Spike Yeongcheon, Korea 340.5 6.8 Gardeniae Fructus Gardenia jasminoides Ellis Rubiaceae Fruit Imsil, Korea 340.5 6.8 Coptidis Rhizoma Coptis japonica Makino Ranunculaceae Rhizome China 340.5 6.8 Aurantii Fructus Citrus aurantium Linné Rutaceae Fruit China 340.5 6.8 Immaturus Mentha arvensis Linné var. Menthae Herba Labiatae Aerial part Uiseong, Korea 340.5 6.8 piperascens Malinvaud ex Holmes Glycyrrhizae Radix et Glycyrrhiza uralensis Fischer Leguminosae Root and rhizome China 204.0 4.1 Rhizoma Total 5000.0 100.0 1 Table 2. Chromatographic conditions for simultaneous quantification of compounds 1–18 in CSBPT. Chromatographic parameter Column SunFire C18 analytical column (250 × 4.6 mm, 5 μm) Detector PDA (235, 250, 280, 310, and 345 nm) Flow rate (mL/min) 1.0 Injection volume (μL) 10.0 Column temperature (°C) 40.0 A: 0.1% (v/v) aqueous formic acid Mobile phase B: 0.1% (v/v) formic acid in acetonitrile Time (min) A (%) B (%) 0 95 5 40 40 60 Gradient elution 50 5 95 55 5 95 60 95 5 70 95 5 2 Table S3. -

Potential Inhibitory Effects of the Traditional Herbal Prescription
MOLECULAR MEDICINE REPORTS 17: 2515-2522, 2018 Potential inhibitory effects of the traditional herbal prescription Hyangso‑san against skin inflammation via inhibition of chemokine production and inactivation of STAT1 in HaCaT keratinocytes HYE‑SUN LIM1, SAE‑ROM YOO2, MEE‑YOUNG LEE2, CHANG‑SEOB SEO2, HYEUN‑KYOO SHIN2 and SOO‑JIN JEONG1,3 1Herbal Medicine Research Division; 2K‑herb Research Center, Korea Institute of Oriental Medicine, Yuseong, Daejeon 34054; 3Korean Medicine Life Science, University of Science and Technology, Yuseong, Daejeon 34113, Republic of Korea Received June 15, 2016; Accepted April 20, 2017 DOI: 10.3892/mmr.2017.8172 Abstract. Inflammatory skin disease are caused by multiple treatment inhibited TNF‑α‑ and IFN‑γ‑induced STAT1 factors, including susceptibility genes, and immunologic and activation. Results from the present study indicated that HSS environmental factors, and are characterized by an increase exhibited inhibitory effects on TNF‑α‑ and IFN‑γ‑mediated in epidermal thickness and the infiltration of macrophages, chemokine production and expression by targeting STAT1 in keratinocytes, mast cells, eosinophils and other inflammatory keratinocytes. Overall, the results indicated that HSS may be cells. Keratinocytes may serve an important role in the patho- a potential candidate therapeutic drug for inflammatory skin genesis of inflammatory skin diseases. The traditional herbal diseases such as atopic dermatitis. decoction Hyangso‑san (HSS) has been used to treat symp- toms of the common cold, including headache, pantalgia, fever Introduction and chills. However, to the best of our knowledge, there is no evidence regarding whether HSS has an effect on inflamma- Skin inflammation is the most common complaint in derma- tory skin diseases. -

Simultaneous Determination of Nine Bioactive
Accepted Manuscript Title: Simultaneous determination of nine bioactive compounds in Yijin-tang via high-performance liquid chromatography and liquid chromatography-electrospray ionization-mass spectrometry Author: Hye Jin Yang Nam-Hui Yim Kwang Jin Lee Min Jung Gu Bohyoung Lee Youn-Hwan Hwang Jin Yeul Ma PII: S2213-4220(16)30030-0 DOI: http://dx.doi.org/doi:10.1016/j.imr.2016.04.005 Reference: IMR 200 To appear in: Received date: 14-10-2015 Revised date: 30-3-2016 Accepted date: 7-4-2016 Please cite this article as: Hye Jin YangNam-Hui YimKwang Jin LeeMin Jung GuBohyoung LeeYoun-Hwan HwangJin Yeul Ma Simultaneous determination of nine bioactive compounds in Yijin-tang via high-performance liquid chromatography and liquid chromatography-electrospray ionization-mass spectrometry (2016), http://dx.doi.org/10.1016/j.imr.2016.04.005 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 Original Article 2 3 Simultaneous determination of nine bioactive compounds in Yijin-tang via high- 4 performance liquid chromatography and liquid chromatography-electrospray 5 ionization-mass spectrometry 6 7 Hye Jin Yang, Nam-Hui Yim, Kwang Jin Lee, Min Jung Gu, Bohyoung Lee, Youn-Hwan * * 8 Hwang and Jin Yeul Ma 9 10 KM Application Center, Korea Institute of Oriental Medicine, 11 70 Cheomdanro, Dong-gu, Daegu 701-300, Republic of Korea 12 13 Running title: Simultaneous determination of Yijin-tang via HPLC-DAD and LC/MS 14 * 15 Address for co-corresponding author 16 Youn-Hwan Hwang, Ph.D.