Spectral Irradiance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Lecture 3: the Sensor
4.430 Daylighting Human Eye ‘HDR the old fashioned way’ (Niemasz) Massachusetts Institute of Technology ChriChristoph RstophReeiinhartnhart Department of Architecture 4.4.430 The430The SeSensnsoror Building Technology Program Happy Valentine’s Day Sun Shining on a Praline Box on February 14th at 9.30 AM in Boston. 1 Happy Valentine’s Day Falsecolor luminance map Light and Human Vision 2 Human Eye Outside view of a human eye Ophtalmogram of a human retina Retina has three types of photoreceptors: Cones, Rods and Ganglion Cells Day and Night Vision Photopic (DaytimeVision): The cones of the eye are of three different types representing the three primary colors, red, green and blue (>3 cd/m2). Scotopic (Night Vision): The rods are repsonsible for night and peripheral vision (< 0.001 cd/m2). Mesopic (Dim Light Vision): occurs when the light levels are low but one can still see color (between 0.001 and 3 cd/m2). 3 VisibleRange Daylighting Hanbook (Reinhart) The human eye can see across twelve orders of magnitude. We can adapt to about 10 orders of magnitude at a time via the iris. Larger ranges take time and require ‘neural adaptation’. Transition Spaces Outside Atrium Circulation Area Final destination 4 Luminous Response Curve of the Human Eye What is daylight? Daylight is the visible part of the electromagnetic spectrum that lies between 380 and 780 nm. UV blue green yellow orange red IR 380 450 500 550 600 650 700 750 wave length (nm) 5 Photometric Quantities Characterize how a space is perceived. Illuminance Luminous Flux Luminance Luminous Intensity Luminous Intensity [Candela] ~ 1 candela Courtesy of Matthew Bowden at www.digitallyrefreshing.com. -
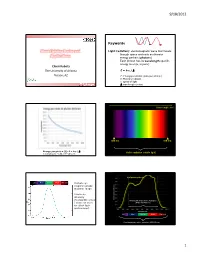
Plant Lighting Basics and Applications
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Fundametals of Rendering - Radiometry / Photometry
Fundametals of Rendering - Radiometry / Photometry “Physically Based Rendering” by Pharr & Humphreys •Chapter 5: Color and Radiometry •Chapter 6: Camera Models - we won’t cover this in class 782 Realistic Rendering • Determination of Intensity • Mechanisms – Emittance (+) – Absorption (-) – Scattering (+) (single vs. multiple) • Cameras or retinas record quantity of light 782 Pertinent Questions • Nature of light and how it is: – Measured – Characterized / recorded • (local) reflection of light • (global) spatial distribution of light 782 Electromagnetic spectrum 782 Spectral Power Distributions e.g., Fluorescent Lamps 782 Tristimulus Theory of Color Metamers: SPDs that appear the same visually Color matching functions of standard human observer International Commision on Illumination, or CIE, of 1931 “These color matching functions are the amounts of three standard monochromatic primaries needed to match the monochromatic test primary at the wavelength shown on the horizontal scale.” from Wikipedia “CIE 1931 Color Space” 782 Optics Three views •Geometrical or ray – Traditional graphics – Reflection, refraction – Optical system design •Physical or wave – Dispersion, interference – Interaction of objects of size comparable to wavelength •Quantum or photon optics – Interaction of light with atoms and molecules 782 What Is Light ? • Light - particle model (Newton) – Light travels in straight lines – Light can travel through a vacuum (waves need a medium to travel in) – Quantum amount of energy • Light – wave model (Huygens): electromagnetic radiation: sinusiodal wave formed coupled electric (E) and magnetic (H) fields 782 Nature of Light • Wave-particle duality – Light has some wave properties: frequency, phase, orientation – Light has some quantum particle properties: quantum packets (photons). • Dimensions of light – Amplitude or Intensity – Frequency – Phase – Polarization 782 Nature of Light • Coherence - Refers to frequencies of waves • Laser light waves have uniform frequency • Natural light is incoherent- waves are multiple frequencies, and random in phase. -

Light and Illumination
ChapterChapter 3333 -- LightLight andand IlluminationIllumination AAA PowerPointPowerPointPowerPoint PresentationPresentationPresentation bybyby PaulPaulPaul E.E.E. Tippens,Tippens,Tippens, ProfessorProfessorProfessor ofofof PhysicsPhysicsPhysics SouthernSouthernSouthern PolytechnicPolytechnicPolytechnic StateStateState UniversityUniversityUniversity © 2007 Objectives:Objectives: AfterAfter completingcompleting thisthis module,module, youyou shouldshould bebe ableable to:to: •• DefineDefine lightlight,, discussdiscuss itsits properties,properties, andand givegive thethe rangerange ofof wavelengthswavelengths forfor visiblevisible spectrum.spectrum. •• ApplyApply thethe relationshiprelationship betweenbetween frequenciesfrequencies andand wavelengthswavelengths forfor opticaloptical waves.waves. •• DefineDefine andand applyapply thethe conceptsconcepts ofof luminousluminous fluxflux,, luminousluminous intensityintensity,, andand illuminationillumination.. •• SolveSolve problemsproblems similarsimilar toto thosethose presentedpresented inin thisthis module.module. AA BeginningBeginning DefinitionDefinition AllAll objectsobjects areare emittingemitting andand absorbingabsorbing EMEM radiaradia-- tiontion.. ConsiderConsider aa pokerpoker placedplaced inin aa fire.fire. AsAs heatingheating occurs,occurs, thethe 1 emittedemitted EMEM waveswaves havehave 2 higherhigher energyenergy andand 3 eventuallyeventually becomebecome visible.visible. 4 FirstFirst redred .. .. .. thenthen white.white. LightLightLight maymaymay bebebe defineddefineddefined -

TIE-35: Transmittance of Optical Glass
DATE October 2005 . PAGE 1/12 TIE-35: Transmittance of optical glass 0. Introduction Optical glasses are optimized to provide excellent transmittance throughout the total visible range from 400 to 800 nm. Usually the transmittance range spreads also into the near UV and IR regions. As a general trend lowest refractive index glasses show high transmittance far down to short wavelengths in the UV. Going to higher index glasses the UV absorption edge moves closer to the visible range. For highest index glass and larger thickness the absorption edge already reaches into the visible range. This UV-edge shift with increasing refractive index is explained by the general theory of absorbing dielectric media. So it may not be overcome in general. However, due to improved melting technology high refractive index glasses are offered nowadays with better blue-violet transmittance than in the past. And for special applications where best transmittance is required SCHOTT offers improved quality grades like for SF57 the grade SF57 HHT. The aim of this technical information is to give the optical designer a deeper understanding on the transmittance properties of optical glass. 1. Theoretical background A light beam with the intensity I0 falls onto a glass plate having a thickness d (figure 1-1). At the entrance surface part of the beam is reflected. Therefore after that the intensity of the beam is Ii0=I0(1-r) with r being the reflectivity. Inside the glass the light beam is attenuated according to the exponential function. At the exit surface the beam intensity is I i = I i0 exp(-kd) [1.1] where k is the absorption constant. -

Reflectance and Transmittance Sphere
Reflectance and Transmittance Sphere Spectral Evolution four inch portable reflectance/ transmittance sphere is ideal for measuring vegetation physiological states SPECTRAL EVOLUTION offers a portable, compact four inch reflectance/transmittance (R/T) integrating sphere for measuring the reflectance and transmittance of vegetation. The 4 inch R/T sphere is lightweight and portable so you can take it into the field for in situ meas- urements, delivered with a stand as well as a ¼-20 mount for use with tripods. When used with a SPECTRAL EVOLUTION spectrometer or spectroradiometer such as the PSR+, RS-3500, RS- 5400, SR-6500 or RS-8800, it delivers detailed information for measurements of transmittance and reflectance modes. Two varying light intensity levels (High and Low) allow for a broader range of samples to be measured. Light reflected or transmitted from a sample in a sphere is integrated over a full hemisphere, with measurements insensitive to sample anisotropic directional reflectance (transmittance). This provides repeatable measurements of a variety of samples. The 4 inch portable R/T sphere can be used in vegetation studies such as, deriving absorbance characteristics, and estimating the leaf area index (LAI) from radiation reflected from a canopy surface. The sphere allows researchers to limit destructive sampling and provides timely data acquisition of key material samples. The R/T sphere allows you to collect all diffuse light reflected from a sample – measuring total hemispherical reflectance. You can measure reflectance and transmittance, and calculate absorption. You can select the bulb inten- sity level that suits your sample. A white reference is provided for both reflectance and transmittance measurements. -

Illumination and Distance
PHYS 1400: Physical Science Laboratory Manual ILLUMINATION AND DISTANCE INTRODUCTION How bright is that light? You know, from experience, that a 100W light bulb is brighter than a 60W bulb. The wattage measures the energy used by the bulb, which depends on the bulb, not on where the person observing it is located. But you also know that how bright the light looks does depend on how far away it is. That 100W bulb is still emitting the same amount of energy every second, but if you are farther away from it, the energy is spread out over a greater area. You receive less energy, and perceive the light as less bright. But because the light energy is spread out over an area, it’s not a linear relationship. When you double the distance, the energy is spread out over four times as much area. If you triple the distance, the area is nine Twice the distance, ¼ as bright. Triple the distance? 11% as bright. times as great, meaning that you receive only 1/9 (or 11%) as much energy from the light source. To quantify the amount of light, we will use units called lux. The idea is simple: energy emitted per second (Watts), spread out over an area (square meters). However, a lux is not a W/m2! A lux is a lumen per m2. So, what is a lumen? Technically, it’s one candela emitted uniformly across a solid angle of 1 steradian. That’s not helping, is it? Examine the figure above. The source emits light (energy) in all directions simultaneously. -

About SI Units
Units SI units: International system of units (French: “SI” means “Système Internationale”) The seven SI base units are: Mass: kg Defined by the prototype “standard kilogram” located in Paris. The standard kilogram is made of Pt metal. Length: m Originally defined by the prototype “standard meter” located in Paris. Then defined as 1,650,763.73 wavelengths of the orange-red radiation of Krypton86 under certain specified conditions. (Official definition: The distance traveled by light in vacuum during a time interval of 1 / 299 792 458 of a second) Time: s The second is defined as the duration of a certain number of oscillations of radiation coming from Cs atoms. (Official definition: The second is the duration of 9,192,631,770 periods of the radiation of the hyperfine- level transition of the ground state of the Cs133 atom) Current: A Defined as the current that causes a certain force between two parallel wires. (Official definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10-7 Newton per meter of length. Temperature: K One percent of the temperature difference between boiling point and freezing point of water. (Official definition: The Kelvin, unit of thermodynamic temperature, is the fraction 1 / 273.16 of the thermodynamic temperature of the triple point of water. Amount of substance: mol The amount of a substance that contains Avogadro’s number NAvo = 6.0221 × 1023 of atoms or molecules. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

Black Body Radiation and Radiometric Parameters
Black Body Radiation and Radiometric Parameters: All materials absorb and emit radiation to some extent. A blackbody is an idealization of how materials emit and absorb radiation. It can be used as a reference for real source properties. An ideal blackbody absorbs all incident radiation and does not reflect. This is true at all wavelengths and angles of incidence. Thermodynamic principals dictates that the BB must also radiate at all ’s and angles. The basic properties of a BB can be summarized as: 1. Perfect absorber/emitter at all ’s and angles of emission/incidence. Cavity BB 2. The total radiant energy emitted is only a function of the BB temperature. 3. Emits the maximum possible radiant energy from a body at a given temperature. 4. The BB radiation field does not depend on the shape of the cavity. The radiation field must be homogeneous and isotropic. T If the radiation going from a BB of one shape to another (both at the same T) were different it would cause a cooling or heating of one or the other cavity. This would violate the 1st Law of Thermodynamics. T T A B Radiometric Parameters: 1. Solid Angle dA d r 2 where dA is the surface area of a segment of a sphere surrounding a point. r d A r is the distance from the point on the source to the sphere. The solid angle looks like a cone with a spherical cap. z r d r r sind y r sin x An element of area of a sphere 2 dA rsin d d Therefore dd sin d The full solid angle surrounding a point source is: 2 dd sind 00 2cos 0 4 Or integrating to other angles < : 21cos The unit of solid angle is steradian. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object.