Contamination Status of Polychlorinated Biphenyls
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rule 391-3-6-.03. Water Use Classifications and Water Quality Standards
Presented below are water quality standards that are in effect for Clean Water Act purposes. EPA is posting these standards as a convenience to users and has made a reasonable effort to assure their accuracy. Additionally, EPA has made a reasonable effort to identify parts of the standards that are not approved, disapproved, or are otherwise not in effect for Clean Water Act purposes. Rule 391-3-6-.03. Water Use Classifications and Water Quality Standards ( 1) Purpose. The establishment of water quality standards. (2) W ate r Quality Enhancement: (a) The purposes and intent of the State in establishing Water Quality Standards are to provide enhancement of water quality and prevention of pollution; to protect the public health or welfare in accordance with the public interest for drinking water supplies, conservation of fish, wildlife and other beneficial aquatic life, and agricultural, industrial, recreational, and other reasonable and necessary uses and to maintain and improve the biological integrity of the waters of the State. ( b) The following paragraphs describe the three tiers of the State's waters. (i) Tier 1 - Existing instream water uses and the level of water quality necessary to protect the existing uses shall be maintained and protected. (ii) Tier 2 - Where the quality of the waters exceed levels necessary to support propagation of fish, shellfish, and wildlife and recreation in and on the water, that quality shall be maintained and protected unless the division finds, after full satisfaction of the intergovernmental coordination and public participation provisions of the division's continuing planning process, that allowing lower water quality is necessary to accommodate important economic or social development in the area in which the waters are located. -

Guidelines for Eating Fish from Georgia Waters 2017
Guidelines For Eating Fish From Georgia Waters 2017 Georgia Department of Natural Resources 2 Martin Luther King, Jr. Drive, S.E., Suite 1252 Atlanta, Georgia 30334-9000 i ii For more information on fish consumption in Georgia, contact the Georgia Department of Natural Resources. Environmental Protection Division Watershed Protection Branch 2 Martin Luther King, Jr. Drive, S.E., Suite 1152 Atlanta, GA 30334-9000 (404) 463-1511 Wildlife Resources Division 2070 U.S. Hwy. 278, S.E. Social Circle, GA 30025 (770) 918-6406 Coastal Resources Division One Conservation Way Brunswick, Ga. 31520 (912) 264-7218 Check the DNR Web Site at: http://www.gadnr.org For this booklet: Go to Environmental Protection Division at www.gaepd.org, choose publications, then fish consumption guidelines. For the current Georgia 2015 Freshwater Sport Fishing Regulations, Click on Wild- life Resources Division. Click on Fishing. Choose Fishing Regulations. Or, go to http://www.gofishgeorgia.com For more information on Coastal Fisheries and 2015 Regulations, Click on Coastal Resources Division, or go to http://CoastalGaDNR.org For information on Household Hazardous Waste (HHW) source reduction, reuse options, proper disposal or recycling, go to Georgia Department of Community Affairs at http://www.dca.state.ga.us. Call the DNR Toll Free Tip Line at 1-800-241-4113 to report fish kills, spills, sewer over- flows, dumping or poaching (24 hours a day, seven days a week). Also, report Poaching, via e-mail using [email protected] Check USEPA and USFDA for Federal Guidance on Fish Consumption USEPA: http://www.epa.gov/ost/fishadvice USFDA: http://www.cfsan.fda.gov/seafood.1html Image Credits:Covers: Duane Raver Art Collection, courtesy of the U.S. -

The Georgia Coast Saltwater Paddle Trail
2010 The Georgia Coast Saltwater Paddle Trail This project was funded in part by the Coastal Management Program of the Georgia Department of Natural Resources, and the U.S. Department of Commerce, Office of Ocean and Coastal Resource Management (OCRM), National Oceanic and Atmospheric Administration (NOAA) grant award #NA09NOS4190171, as well as the National Park Service Rivers, Trails & Conservation Assistance Program. The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of OCRM or NOAA. September 30, 2010 0 CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................................... 2 Coastal Georgia Regional Development Center Project Team .......................................................... 3 Planning and Government Services Staff ................................................................................................... 3 Geographic Information Systems Staff ....................................................................................................... 3 Economic Development Staff .......................................................................................................................... 3 Administrative Services Staff .......................................................................................................................... 3 Introduction ............................................................................................................................................................... -
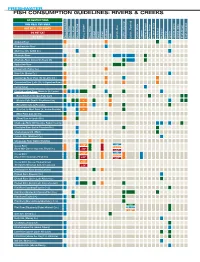
Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Lotts Crk.) Canoochee River (Lotts Crk. to Ogeechee River) Casey Canal Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Chickamauga Crk. (West) Cohulla Crk. (Whitfield Co.) Conasauga River (below Stateline) <18" Coosa River <20" 18 –32" (River Mile Zero to Hwy 100, Floyd Co.) ≥20" >32" <18" Coosa River <20" 18 –32" (Hwy 100 to Stateline, Floyd Co.) ≥20" >32" Coosa River (Coosa, Etowah below <20" Thompson-Weinman dam, Oostanaula) ≥20" Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. -

A Visitor's Guide to Accessing Georgia's Coastal Resources
A Visitor’s Guide to Accessing Georgia’s Coastal Resources Beaches & Barrier Islands Cultural & Historic Sites Rivers & Waterways Wildlife Viewing & Walking Trails FREE COPY - NOT FOR SALE A Visitor’s Guide to Accessing Georgia’s Coastal Resources acknowledgements This Guide was prepared by The University of Georgia Marine Extension Service under grant award # NA06NOS4190253 from the Office of Ocean and Coastal Resource Management, National Oceanic and Atmospheric Administration. The statements, findings, conclusions, and recommendations are those of the author(s) and do not necessarily reflect the views of OCRM or NOAA. The authors gratefully acknowledge the Georgia Department of Natural Resources’ Wildlife Resources Division and Parks and Historic Sites Division for their assistance and for permission to use certain descriptions, maps, and photographs in the drafting of this Guide. The authors also acknowledge the Coastal Resources Division and particularly Beach Water Quality Manager Elizabeth Cheney for providing GIS maps and other helpful assistance related to accessing Georgia beaches. This Access Guide was compiled and written by Phillip Flournoy and Casey Sanders. University of Georgia Marine Extension Service 715 Bay Street Brunswick, GA 31520 April 2008 Photo Credits: ~ Beak to Beak Egret Chicks by James Holland, Altamaha Riverkeeper ~ Sapelo Island Beach by Suzanne Van Parreren, Sapelo Island National Estuarine Research Reserve ~ Main House, Hofwyl Plantation by Robert Overman, University of Georgia Marine Extension Service ~ J. T. Good, A Chip Off the Block by Captain Brooks Good table of contents Acknowledgements. 2 Map of Georgia Coastal Counties and the Barrier Islands. 5 Foreword. 6 1. Beaches and Barrier Islands . 7 a. Chatham County. -

2014 Coastal Streams
2014 Integrated 305(b)/303(d) List Coastal Streams - Supporting Designated Uses Reach Name/ ID #/ Reach Location/ River Basin/ Criterion Potential Data Source County Use Violated Causes Extent Category Priority Notes Altamaha River Butler River to Altamaha Sound Altamaha 7 miles 1 R030701060509 Glynn/ McIntosh County Fishing 1,5,55 Bungalow Creek Headwaters to Hampton River Altamaha 2 miles 1 R030701060519 Glynn County Fishing 5 Butler River Altamaha River to Altamaha River Altamaha 5 miles 1 (upstream and downstream of I-95) R030701060504 McIntosh County Fishing 5 Darien River Cathead Creek to May Hall Creek Altamaha 5 miles 1 (formerly Cathead Creek to May Creek) R030701060511 McIntosh County Fishing 1,5,55 Hampton River Village Creek to Bungalow Creek Altamaha 1 miles 1 R030701060521 Glynn County Fishing 5 Hampton River Mosquito Creek to Village Creek Altamaha 3 miles 1 R030701060516 Glynn County Fishing 5 Pine Creek Hampton River to the Hampton River Altamaha 2 miles 1 R030701060517 Glynn County Fishing 5 A-326 2014 Integrated 305(b)/303(d) List Coastal Streams - Supporting Designated Uses Reach Name/ ID #/ Reach Location/ River Basin/ Criterion Potential Data Source County Use Violated Causes Extent Category Priority Notes South Altamaha River Altamaha River to Buttermilk Sound Altamaha 15 miles 1 R030701060505 McIntosh/ Glynn County Fishing 5 South Branch (aka Altamaha River to Altamaha River Altamaha 5 miles 1 South Altamaha River) near Cambers Island R030701060510 McIntosh/ Glynn County Fishing 5 Village Creek Bend in Creek at Village Drive to Altamaha 3 miles 1 Hampton River R030701060518 Glynn County Fishing 5 Barbour Island River Wahoo River to Sapelo Sound Ogeechee 8 miles 1 R030602040701 McIntosh County Fishing 5 Bear River Killkenny Creek to St. -

River Highway for Trade, the Savannah : Canoes, Indian Tradeboats
RIVER HIGHWAY FOR TRADE THE SAVANNAH BY RUBY A. RAHN CANOES. INDIAN TRADEBOATS, FLATBOATS, STEAMERS, PACKETS. AND BARGES UG 23 S29 PUBLISHED BY 1968 U. S. ARMY ENGINEER DISTRICT, SAVANNAH CORPS OF ENGINEERS SAVANNAH, GEORGIA JUNE 1968 FOREWORD River Highway for Trade by Ruby A. Rahn is the result of nearly a quarter of a century of research into contemporary newspaper files, old letters, and documents as well as personal memories. Miss Rahn, a long-time school teacher in the school sys tem of Savannah, was born in Effingham County in 1883. She grew up close to the River, during those years when the life and excitement of the River was still a part of local living. Miss Rahn was assisted in the compilation of the monograph by her niece, Naomi Gnann LeBey. The information of the mono graph offers a vivid and valuable record of river activities from the time of Indian habitation through the 19th century. Sometimes supplementary items of the period are included which seem proper in this miscellany of interesting infor mation. M. L. Granger Editor I NTRODUCTI ON I wish to acknowledge with gratitude the help and en couragement received from Mrs. Lilla Hawes, Miss Bessie Lewis, and Mr. Edward Mueller. They were, indeed, friends in my need. The information on the poleboats was all taken from the Marine News reports of the daily newspapers of the time. The totals of cotton bales for these boats can only be ap proximate, as the poleboats were hauling cotton for a few years before the papers started to publish the Marine News. -

Graptemys Barbouri)
Species Status Assessment Report for the Barbour’s Map Turtle (Graptemys barbouri) Adult female Barbour’s map turtle, Chipola River, FL. (credit: Jonathan Mays, FWC) May 2017 U.S. Fish and Wildlife Service Region 4 Atlanta, GA This document was prepared by Lisa Yarbrough (U.S. Fish and Wildlife Service – Panama City, FL Ecological Services Field Office) with assistance from Dr. Sean Blomquist (U.S. Fish and Wildlife Service – Panama City, FL Ecological Services Field Office) and Andreas Moshogianis (U.S. Fish and Wildlife Service – Region 4/Southeast Regional Office). Valuable peer reviews of a draft of this document were provided by John Jensen (Georgia Department of Natural Resources), Jonathan Mays (Florida Fish and Wildlife Conservation Commission), Jim Godwin (Alabama Natural Heritage Program), Lora Smith (Joseph W. Jones Ecological Research Center, Georgia), Sean Sterrett (University of Massachusetts), and Marshall Williams (U.S. Fish and Wildlife Service – Region 4/ Southeast Regional Office). We appreciate the time and effort of those dedicated to learning and implementing the SSA Framework, which resulted in a more robust assessment and final report. Suggested reference: U.S. Fish and Wildlife Service. 2017. Species status assessment report for the Barbour’s Map Turtle (Graptemys barbouri). May, 2017. Atlanta, GA. Barbour’s Map Turtle SSA Page ii 2017 Species Status Assessment Report For Barbour’s Map Turtle (Graptemys barbouri) Prepared by the U.S. Fish and Wildlife Service EXECUTIVE SUMMARY This species status assessment (SSA) reports the results of the comprehensive status review for the Barbour’s map turtle (Graptemys barbouri), documenting the species’ historical condition and providing estimates of current and future condition under a range of different scenarios. -

GDOT Powerpoint Template
Fiscal Year 2016 Letting Report Number of Projects Let in FY 2016 (Contractor Low Bid Award Data) GDOT & Local Lets 3; 7% 3; 7% 1; 2% Bridges Roads Enhancements 10; 23% 26; 61% Maintenance Safety Total = 75 As of September 14, 2015 Does not include LMIG Projects Source Construction Bidding Administration Fiscal Year 2016 Letting Report Dollar Amount of Projects Let in FY 2016 (Contractor Low Bid Award Data) $20,620,692; 8% $30,932,555; 13% GDOT & Local Lets Bridges Roads Enhancements $57,559,224; 24% Maintenance $133,935,878; 55% Safety $967,708; 0% Total = $248,620,259 As of September 14, 2015 Does not include LMIG Projects Source Construction Bidding Administration Summary Presented to Low Bid GDOT Board Low Bid w/Adjustments (Includes Adjustments) (Awarded to Contractor) (Total Project Obligation) GDOT Let 45 43 43 (Project Count) GDOT Let $173,014,184 $136,847,381 $145,184,448 (Dollars) Local Let 4 N/A Local Let N/A Local Let (Project Count) Local Let $4,299,022 N/A Local Let N/A Local Let (Dollars) *Low Bid Award Amount includes the Deferred and Awarded projects. Does not include Engineering and Inspection, Utilities, Liquid AC, Incentives, LMIG and Rejected projects. As of September 14, 2015 Source Construction Bidding Administration GDOT Let Projects October 2015 Letting GDOT Let Projects October 2015 Letting Primary Primary Congressional Project Project Project ID Description County Work Type District Let By Sponsor M005112 I-95 SB & NB @ LITTLE SATILLA RIVER - BRIDGE REHAB CAMDEN Bridges 1 DOT GDOT SR 25 CONN/BAY STREET FROM -

Zone Details
THE DOLPHIN PROJECT Survey Zones NMFS Permit #19088 Code TDP NOAA Narrative Main Waters 1 SA055WRI 11516 One Pass: Skull Creek, "27" North to "3"; MackayCreek, "24" North to "1". Chechessee River to Zigzag Pass: Chechessee River, between N"2"/"1" to a north/south line through Mackay & Skull Creeks a platform at Foot Point 2 SA054 11516 One Pass: Broad Creek, G"1" to G"19"; Northwest side of Marsh Island; Bull Calibogue Sound. May Creek south of May River to 32°11.4'N. Zigzag Pass: Northern portion of River Calibogue Sound, "32"/G"1" to "24"/G"27"; May River westward to "8" 3 SA052CAL 11512 One Pass: Southern portion of Calibogue Sound North boundary a line from Haig Point to "32" to "1". Eastern boundary a line from "1" to Braddoch Point to Calibogue Sound. "4" to "2" to 32°03'3/80°47'7. Southern boundary an EW line at 32°03'3. Western boundary a line from 32°03'3/80°50'3 to Haig Point 4 SA051NWR 11512 One Pass: New River from Bloody Point to 32°09'5/80°57' Mungen Creek; Ramshorn Creek; Cooper River west of "35". Bull Creeknorth of "34"; Unnamed New River Creel East of entrance to Bull Creek when tides allow. Zigzag Pass: Cooper River east of "35" to Haig Point. 5 SA051WRI 11512 One Pass: Wright River, Turbridge Landing to south of Turtle Island 32°03'/80°45'. ICW "48" to "45" Fields Cut & "44" to "42" Walls Cut. Unnamed Wright River Creek north of Walls Cut 6 GA001SAV 11512 Zigzag Pass: Savannah River from Mackay Point on the west to just east of marker "20". -

GDOT Bridge Projects
GDOT Bridge Projects PROJECT ID DESCRIPTION COUNTIES CONSTRUCTION CONSTRUCTION PRELIMINARY PRELIMINARY RIGHT OF RIGHT OF WAY FUNDING ENGINEERING ENGINEERING WAY SOURCE YEAR AMOUNT YEAR AMOUNT YEAR AMOUNT 532290- CR 536/ZOAR ROAD @ BIG SATILIA CREEK TRIBUTARY Appling TBD TBD TBD TBD LOCL $14,850.00 0013818 SR 64 @ SATILLA RIVER 6 MI E OF PEARSON Atkinson 2020 $3,300,000.00 2016 $500,000.00 2019 $250,000.00 Federal 0015581 Bridge Replacement of CR 180 (Liberty Church Road) over Little Hurricane Creek. This Bacon N/A N/A 2019 $250,000.00 N/A N/A Federal bridge is structurally deficient and requires posting as cross bracing has been added at each intermediate bent, some have been replaced and concrete is spalling under deck and exposing rebar. 570720- CR 159 @ LITTLE HURRICANE CREEK NW OF ALMA Bacon TBD TBD TBD TBD LOCL $29,700.00 0007154 The proposed project would consist of replacing the bridge on SR 216 at Baker 2017 $6,454,060.87 2007 $667,568.36 2016 $290,000.00 Federal Ichawaynochaway Creek by closing the existing roadway & maintaining traffic on an off- site detour of approximately 40 miles. this project is located 12.7 miles northwest of Newton, Georgia and is 0.16 miles in length. Bridge ID: 007-0007-0 0007153 This project is the replacement of the existing bridge on SR 200@ Ichawaynochaway Baker 2018 $4,068,564.69 2012 $766,848.95 2017 $70,000.00 State Creek. The current bridge sufficency rating is 55.63 and will be replaced with a wider bridge that meets current GDOT guidelines. -

Bookletchart™ Altamaha Sound NOAA Chart 11508
BookletChart™ Altamaha Sound NOAA Chart 11508 A reduced-scale NOAA nautical chart for small boaters When possible, use the full-size NOAA chart for navigation. Published by the Altamaha River is formed by the confluence of the Oconee River and Ocmulgee River, 110 miles above the town of Darien and 119 miles National Oceanic and Atmospheric Administration above its mouth, and flows in a general southeasterly direction, entering National Ocean Service the western end of Altamaha Sound. The river is subject to freshets, and Office of Coast Survey depths change radically. In 1983, the reported controlling depth was 3 feet during 8 months of www.NauticalCharts.NOAA.gov the year to Milledgeville, a city on the Oconee River 126 miles above the 888-990-NOAA junction with the Altamaha River, and 3 feet to Macon, a city on the Ocmulgee River 178 miles above the junction. The depths are 2 to 12 What are Nautical Charts? feet less during the summer low-water period. U.S. Route 17 highway bridge over South Altamaha River, 2.5 miles Nautical charts are a fundamental tool of marine navigation. They show south of Darien, has a fixed span with a clearance of 35 feet. An water depths, obstructions, buoys, other aids to navigation, and much overhead power cable on the west side of the bridge has a clearance of more. The information is shown in a way that promotes safe and 55 feet. Interstate Route 95 highway bridge crossing South Altamaha efficient navigation. Chart carriage is mandatory on the commercial River, about 1.2 miles westward of U.S.