Graptemys Barbouri)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Federal Register/Vol. 73, No. 232/Tuesday, December
73182 Federal Register / Vol. 73, No. 232 / Tuesday, December 2, 2008 / Rules and Regulations (subtitle E of the Small Business any person acting subject to the SUPPLEMENTARY INFORMATION: The Regulatory Enforcement Fairness Act of direction or control of a foreign Federal Emergency Management Agency 1996). Therefore, the reporting government or official where such (FEMA) makes the final determinations requirement of 5 U.S.C. 801 does not person is an agent of Cuba or any other listed below for the modified BFEs for apply. country that the President determines each community listed. These modified Paperwork Reduction Act (and so reports to the Congress) poses a elevations have been published in threat to the national security interest of newspapers of local circulation and The Paperwork Reduction Act (PRA) the United States for purposes of 18 ninety (90) days have elapsed since that does not apply to this rule change. See U.S.C. 951; or has been convicted of or publication. The Assistant 44 U.S.C. 3501–3521. The PRA imposes entered a plea of nolo contendere to any Administrator of the Mitigation certain protocol for the ‘‘collection of offense under 18 U.S.C. 792–799, 831, Directorate has resolved any appeals information’’ by government agencies. or 2381, or under section 11 of the resulting from this notification. The Act defines the ‘‘collection of Export Administration Act of 1979, 50 This final rule is issued in accordance information’’ as ‘‘the obtaining, causing U.S.C. app. 2410. with section 110 of the Flood Disaster to be obtained, soliciting, or requiring * * * * * Protection Act of 1973, 42 U.S.C. -

Stream-Temperature Characteristics in Georgia
STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA By T.R. Dyar and S.J. Alhadeff ______________________________________________________________________________ U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 96-4203 Prepared in cooperation with GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Atlanta, Georgia 1997 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 3039 Amwiler Road, Suite 130 Denver Federal Center Peachtree Business Center Box 25286 Atlanta, GA 30360-2824 Denver, CO 80225-0286 CONTENTS Page Abstract . 1 Introduction . 1 Purpose and scope . 2 Previous investigations. 2 Station-identification system . 3 Stream-temperature data . 3 Long-term stream-temperature characteristics. 6 Natural stream-temperature characteristics . 7 Regression analysis . 7 Harmonic mean coefficient . 7 Amplitude coefficient. 10 Phase coefficient . 13 Statewide harmonic equation . 13 Examples of estimating natural stream-temperature characteristics . 15 Panther Creek . 15 West Armuchee Creek . 15 Alcovy River . 18 Altamaha River . 18 Summary of stream-temperature characteristics by river basin . 19 Savannah River basin . 19 Ogeechee River basin. 25 Altamaha River basin. 25 Satilla-St Marys River basins. 26 Suwannee-Ochlockonee River basins . 27 Chattahoochee River basin. 27 Flint River basin. 28 Coosa River basin. 29 Tennessee River basin . 31 Selected references. 31 Tabular data . 33 Graphs showing harmonic stream-temperature curves of observed data and statewide harmonic equation for selected stations, figures 14-211 . 51 iii ILLUSTRATIONS Page Figure 1. Map showing locations of 198 periodic and 22 daily stream-temperature stations, major river basins, and physiographic provinces in Georgia. -

Prohibited Waterbodies for Removal of Pre-Cut Timber
PROHIBITED WATERBODIES FOR REMOVAL OF PRE-CUT TIMBER Recovery of pre-cut timber shall be prohibited in those waterbodies that are considered pristine due to water quality or clarity or where the recovery of pre-cut timber will have a negative impact on, or be an interruption to, navigation or recreational pursuits, or significant cultural resources. Recovery shall be prohibited in the following waterbodies or described areas: 1. Alexander Springs Run 2. All Aquatic Preserves designated under chapter 258, F.S. 3. All State Parks designated under chapter 258, F.S. 4. Apalachicola River between Woodruff lock to I-10 during March, April and May 5. Chipola River within state park boundaries 6. Choctawhatchee River from the Alabama Line 3 miles south during the months of March, April and May. 7. Econfina River from Williford Springs south to Highway 388 in Bay County. 8. Escambia River from Chumuckla Springs to a point 2.5 miles south of the springs 9. Ichetucknee River 10. Lower Suwannee River National Refuge 11. Merritt Mill Pond from Blue Springs to Hwy. 90 12. Newnan’s Lake 13. Ocean Pond – Osceola National Forest, Baker County 14. Oklawaha River from the Eureka Dam to confluence with Silver River 15. Rainbow River 16. Rodman Reservoir 17. Santa Fe River, 3 Miles above and below Ginnie Springs 18. Silver River 19. St. Marks from Natural Bridge Spring to confluence with Wakulla River 20. Suwannee River within state park boundaries 21. The Suwannee River from the Interstate 10 bridge north to the Florida Sheriff's Boys Ranch, inclusive of section 4, township 1 south, range 13 east, during the months of March, April and May. -

Corbicula Management Plan
Management Plan for Control of Asian Clam (Corbicula fluminea) in the Mukwonago River Watershed Crooked Creek Preserve, Mukwonago River Watershed (Conservancy, 2014). Kara Henderlight, Jennifer Hoelzer, Kara Kehl, Brian McDonald, Erin Peeters, Jason Tutkowski, and Cory Widmayer December 10, 2014 1 Table of Contents Introduction……………………………………………………………………………..…………3 Background…………………………………………………………………………………….….4 Mukwonago River Watershed…………………………………………………………….4 Native Unionid Mussels……………………………………………………….…………..6 Corbicula fluminea………………………………………………………………………..7 Preliminary Assessment…………………………………………………………………………...8 Problem……………………………………………………………………………………...…….9 Goals and Objectives…………………………………………………………………….………11 Management Strategies…………………………………………………………………………..12 Prevention…………………………………………………………………………..……12 Education and Outreach………………………………………………………….………13 Interventions…...……………………………………………………………………...…………14 Lakes: Benthic Barriers and Suction Harvesting……………………………………..….16 Rivers: Manual Removal……………………………………………………………..….18 No Action Plan………………………………………………………………………..….23 Monitoring and Assessment……………………………………………….……………………..24 Resources………………………………………………………………………………...………25 Conclusion…………………………………………………………………………………...…..26 References…………………………………………………………………………….………….28 Figure References………………………………………………………………………….…….33 Appendix A: Grants…………………………………………………………………….………..34 Appendix B: Businesses………………………………………………………………..………..37 Appendix C: Public and Private Grade Schools………………………………………………...39 Appendix D: Environmental Organizations…………………………………………….……….41 -

The Asiatic Clam Corbicula Fluminea (Müller, 1774)
The Asiatic clam Corbicula fluminea (Müller, 1774) (Bivalvia: Corbiculidae) in Europe R. Araujo, D. Moreno and M. A. Ramos Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal, 2. 28006, Madrid, Spain Abstract. Two populations of Corbicula fluminea were found in the Iberian Península; one in Spain and the other in Portugal. A detailed description in terms of ecology shell morphology and microstructure, morphometrics and anatomy is given for the Spanish population from the Mino River. Lectotypes for Tellina fluminea and T. fluminalis, and a neotype for T. fluviatilis are designated and illustrated. Distribuüon and spread of C. fluminea in Europe are revised. Comparisons among some European populations and the populations from Cantón, China, and the Mino River are made. Results suggest that, except for one doubtful population, all records of Corbicula in Europe are attributable to C. fluminea. Corbicula taxonomy begins in 1774 with Müller who Thus, Talavera and Faustino (1933) {In: Britton and described three species in the genus Tellina Linne, 1758: T. Morton, 1979) placed Corbicula manilensis (Philippi, 1844) fluminalis "in fluvio Asiae Euphrat"; T. fluminea "in arena into synonymy with C. fluminea, Morton (1977) considered fluviali Chinae"; T. fluviatilis "in ilumine emporium Can C. leana to be a júnior synonym of C. fluminea, while C. tón Chinae praeterlabente". Since then, many living species fluviatilis was previously placed into synonymy with C. of Corbicula Mühlfeldt, 1811, have been described in fluminea by Prashad (1929). Moreover, a thorough review by freshwater and estuarine habitáis from Southeast Asia, the Britton and Morton (1979) lead the authors to consider that Indian subcontinent, the Pacific islands, and the easternmost most Asiatic species previously described could be attributed part of Europe and África (McMahon, 1983). -

Movement and Habitat Use of Two Aquatic Turtles (Graptemys Geographica and Trachemys Scripta) in an Urban Landscape
Urban Ecosyst DOI 10.1007/s11252-008-0049-8 Movement and habitat use of two aquatic turtles (Graptemys geographica and Trachemys scripta) in an urban landscape Travis J. Ryan & Christopher A. Conner & Brooke A. Douthitt & Sean C. Sterrett & Carmen M. Salsbury # Springer Science + Business Media, LLC 2008 Abstract Our study focuses on the spatial ecology and seasonal habitat use of two aquatic turtles in order to understand the manner in which upland habitat use by humans shapes the aquatic activity, movement, and habitat selection of these species in an urban setting. We used radiotelemetry to follow 15 female Graptemys geographica (common map turtle) and each of ten male and female Trachemys scripta (red-eared slider) living in a man-made canal within a highly urbanized region of Indianapolis, IN, USA. During the active season (between May and September) of 2002, we located 33 of the 35 individuals a total of 934 times and determined the total range of activity, mean movement, and daily movement for each individuals. We also analyzed turtle locations relative to the upland habitat types (commercial, residential, river, road, woodlot, and open) surrounding the canal and determined that the turtles spent a disproportionate amount of time in woodland and commercial habitats and avoided the road-associated portions of the canal. We also located 21 of the turtles during hibernation (February 2003), and determined that an even greater proportion of individuals hibernated in woodland-bordered portions of the canal. Our results clearly indicate that turtle habitat selection is influenced by human activities; sound conservation and management of turtle populations in urban habitats will require the incorporation of spatial ecology and habitat use data. -
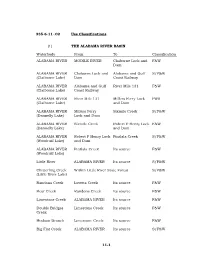
11-1 335-6-11-.02 Use Classifications. (1) the ALABAMA RIVER BASIN Waterbody from to Classification ALABAMA RIVER MOBILE RIVER C
335-6-11-.02 Use Classifications. (1) THE ALABAMA RIVER BASIN Waterbody From To Classification ALABAMA RIVER MOBILE RIVER Claiborne Lock and F&W Dam ALABAMA RIVER Claiborne Lock and Alabama and Gulf S/F&W (Claiborne Lake) Dam Coast Railway ALABAMA RIVER Alabama and Gulf River Mile 131 F&W (Claiborne Lake) Coast Railway ALABAMA RIVER River Mile 131 Millers Ferry Lock PWS (Claiborne Lake) and Dam ALABAMA RIVER Millers Ferry Sixmile Creek S/F&W (Dannelly Lake) Lock and Dam ALABAMA RIVER Sixmile Creek Robert F Henry Lock F&W (Dannelly Lake) and Dam ALABAMA RIVER Robert F Henry Lock Pintlala Creek S/F&W (Woodruff Lake) and Dam ALABAMA RIVER Pintlala Creek Its source F&W (Woodruff Lake) Little River ALABAMA RIVER Its source S/F&W Chitterling Creek Within Little River State Forest S/F&W (Little River Lake) Randons Creek Lovetts Creek Its source F&W Bear Creek Randons Creek Its source F&W Limestone Creek ALABAMA RIVER Its source F&W Double Bridges Limestone Creek Its source F&W Creek Hudson Branch Limestone Creek Its source F&W Big Flat Creek ALABAMA RIVER Its source S/F&W 11-1 Waterbody From To Classification Pursley Creek Claiborne Lake Its source F&W Beaver Creek ALABAMA RIVER Extent of reservoir F&W (Claiborne Lake) Beaver Creek Claiborne Lake Its source F&W Cub Creek Beaver Creek Its source F&W Turkey Creek Beaver Creek Its source F&W Rockwest Creek Claiborne Lake Its source F&W Pine Barren Creek Dannelly Lake Its source S/F&W Chilatchee Creek Dannelly Lake Its source S/F&W Bogue Chitto Creek Dannelly Lake Its source F&W Sand Creek Bogue -

Common Name: ALABAMA MAP TURTLE Scientific Name: Graptemys Pulchra Baur Other Commonly Used Names: Alabama Sawback Previously
Common Name: ALABAMA MAP TURTLE Scientific Name: Graptemys pulchra Baur Other Commonly Used Names: Alabama sawback Previously Used Scientific Names: none Family: Emydidae Rarity Ranks: G4/S1 State Legal Status: Rare Federal Legal Status: none Description: The Alabama map turtle reaches a maximum carapace length of 27.3 cm (10¾ inches). The medially-keeled carapace has a series of laterally compressed spines which are especially pronounced posteriorly. A narrow dark stripe extends along the length of the keel, though it may be interrupted. The majority of the carapace is olive-colored with faint yellow reticulations. Relatively conspicuous concentric yellow markings are found on the dorsal surface of the marginal scutes. The pale yellow plastron has dark markings along the seams, and the ventral surface of the marginals is patterned with a set of concentric dark rings. The skin is dark brown or olive and has many light-green or yellow stripes. A large yellow or light-green patch or "mask" is present on the head, between and behind the eyes. Adult females are much larger than males and have greatly enlarged heads. Similar Species: Common map turtles (Graptemys geographica) may be found in association with Alabama map turtles within the Coosa River drainage, but the latter can be distinguished by their prominent spiny keel on the carapace (except old adult females) and the large yellow or light-green blotch present on the head between and behind the eyes. Habitat: This species prefers large streams or rivers with swiftly flowing water and an abundance of basking sites in the form of fallen trees or snags. -

State-Designated Paddling Trails Paddling Guides
State-Designated Paddling Trails Paddling Guides Compiled from (http://www.dep.state.fl.us/gwt/guide/paddle.htm) This paddling guide can be downloaded at http://www.naturalnorthflorida.com/download-center/ Last updated March 16, 2016 The Original Florida Tourism Task Force 2009 NW 67th Place Gainesville, FL 32653-1603 352.955.2200 ∙ 877.955.2199 Table of Contents Chapter Page Florida’s Designated Paddling Trails 1 Aucilla River 3 Ichetucknee River 9 Lower Ochlockonee River 13 Santa Fe River 23 Sopchoppy River 29 Steinhatchee River 39 Wacissa River 43 Wakulla River 53 Withlacoochee River North 61 i ii Florida’s Designated Paddling Trails From spring-fed rivers to county blueway networks to the 1515-mile Florida Circumnavigational Saltwater Paddling Trail, Florida is endowed with exceptional paddling trails, rich in wildlife and scenic beauty. If you want to explore one or more of the designated trails, please read through the following descriptions, click on a specific trail on our main paddling trail page for detailed information, and begin your adventure! The following maps and descriptions were compiled from the Florida Department of Environmental Protection and the Florida Office of Greenways and Trails. It was last updated on March 16, 2016. While we strive to keep our information current, the most up-to-date versions are available on the OGT website: http://www.dep.state.fl.us/gwt/guide/paddle.htm The first Florida paddling trails were designated in the early 1970s, and trails have been added to the list ever since. Total mileage for the state-designated trails is now around 4,000 miles. -

Economic Importance and Public Preferences for Water Resource Management of the Ocklawaha River
Economic Importance and Public Preferences for Water Resource Management of the Ocklawaha River Tatiana Borisova ([email protected] ), Xiang Bi ([email protected]), Alan Hodges ([email protected]) Food and Resource Economics Department, and Stephen Holland ([email protected] ) Department of Tourism, Recreation, and Sport Management, University of Florida November 11, 2017 Photo of the Ocklawaha River near Eureka West Landing; March 2017 (credit: Tatiana Borisova) Ocklawaha River: Economic Importance and Public Preferences for Water Resource Management Tatiana Borisova ([email protected] ), Xiang Bi ([email protected]), Alan Hodges ([email protected]) Food and Resource Economics Department, Stephen Holland ([email protected] ) Department of Tourism, Recreation, and Sport Management, University of Florida Acknowledgements: Funding for this project was provided by the following organizations: Silver Springs Alliance, Florida Defenders of the Environment, Putnam County Environmental Council, Suwannee-St. Johns Sierra Club, Marion County Soil and Water Conservation District, St. Johns Riverkeeper, Sierra Club Foundation, and Felburn Foundation. We appreciate vehicle counter data for several locations in the study area shared by the Office of Greenways and Trails (Florida Department of Environmental Protection) and Marion County Parks and Recreation. The Florida Survey Research Center at the University of Florida designed the visitor interview questionnaire, and conducted the survey interviews with visitors. Finally, we are grateful to all -

Strategic Habitat and River Reach Units for Aquatic Species of Conservation Concern in Alabama by E
GEOLOGICAL SURVEY OF ALABAMA SPECIAL MAP 248 Strategic Habitat and River Reach Units for Aquatic Species of Conservation Concern in Alabama by E. Anne Wynn, Patrick E. O'Neil, and Stuart W. McGregor - Geological Survey of Alabama; Jeffrey R. Powell, Jennifer M. Pritchett, and Anthony D. Ford - U.S. Fish and Wildlife Service; Berry H. (Nick) Tew, Jr. State Geologist and Paul D. Johnson - Alabama Department of Conservation and Natural Resources TENNESSEE Explanation Rivers and streams 4 Open water County lines 3 5 8 9 Hydrologic Unit Code (HUC) 10 LAUDERDALE 6 subregion boundary LIMESTONE Strategic Habitat Unit (SHU) 2 39 MADISON JACKSON COLBERT Strategic River Reach Unit (SRRU) LAWRENCE FRANKLIN 7 MORGAN The U.S. Fish and Wildlife Service DE KALB 1 MARSHALL in conjunction with the Alabama 20 22 CHEROKEE Department of Conservation and MARION Natural Resources and the Geological 18 37 CULLMAN GEORGIA Survey of Alabama have selected WINSTON BLOUNT ETOWAH watersheds and river segments in the five 19 38 major HUC 4 subregions in Alabama to focus 23 36 conservation activities for managing, recovering, FAYETTE WALKER LAMAR ST CLAIR 35 and restoring populations of rare fishes, mussels, 21 41 CALHOUN snails, and crayfishes. These Strategic Habitat Units 17 CLEBURNE 14 (SHUs) and Strategic River Reach Units (SRRUs) 32 33 JEFFERSON include a substantial part of Alabama’s remaining 16 34 high-quality water courses and reflect the variety of aquatic 31 RANDOLPH habitats occupied by these species historically and presently. MISSISSIPPI 30 PICKENS 15 The SHUs were selected based on the presence of federally CLAY SHELBY listed and state imperiled species, potential threats to the TUSCALOOSA TALLADEGA 27 species, designation of critical habitat, and the best available 29 TALLAPOOSA information about the essential habitat components required by 13 BIBB CHAMBERS these aquatic species to survive. -

COMMON MAP TURTLE Scientific Name: Graptemys Geographica Le
Common Name: COMMON MAP TURTLE Scientific Name: Graptemys geographica Le Sueur Other Commonly Used Names: none Previously Used Scientific Names: none Family: Emydidae Rarity Ranks: G5/S1 State Legal Status: Rare Federal Legal Status: none Description: Like other species of map turtles, the common map turtle exhibits strong sexual dimorphism in both total size and head size. While males reach a carapace length of only 15 cm (6 inches), females may attain a carapace length of up to 27 cm (10½ inches). In addition, females have a much broader head than do males. The carapace is olive-green, with fine, lighter green or yellow lines that form a reticulated pattern, similar in appearance to the contour lines of a topographic map. A low, vertebral keel is present, though not nearly as prominent as the keel found on other species of map turtles. Absent on adult females, low vertebral spines may be evident on the posterior portion of the carapace in both juveniles and adult males. The cream to yellow plastron is unmarked in adults, though the bridge and lower marginal scutes have longitudinal dark lines and circular dark markings, respectively. The skin is olive to dark-brown or black with many narrow yellow to light green stripes. Behind each eye is a small yet distinctive yellow spot, not connected to any of the stripes. Juveniles have dark markings along the seams of the plastron and a more intricate carapace pattern. Similar Species: Common map turtles may be found in association with Alabama map turtles (Graptemys pulchra) within the Coosa River drainage, but the latter can be distinguished by their prominent spiny keel on the carapace (except old adult females) and the large yellow or light-green blotch present on the head between and behind the eyes.