A Case Study of Johnson & Johnson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Get to the Heart of Pain Relief
Get to the heart of pain relief Explain why analgesic choice matters to patients with, or at risk for, cardiovascular disease Counseling tips | Dosage | Savings Analgesic considerations that matter to the heart Reinforce your TYLENOL® recommendation to patients with, or at risk for, cardiovascular disease with these counseling tips: TYLENOL® won’t increase the risk of heart attack, heart failure, or stroke the way ibuprofen or naproxen sodium can1 TYLENOL® won’t interfere with aspirin heart therapy the way ibuprofen can2 TYLENOL® won’t increase blood pressure like NSAIDs sometimes can3,4 TYLENOL® won’t interfere with certain high blood pressure medications, such as diuretics and ACE inhibitors, the way NSAIDs sometimes can3,4 Order patient education, samples, and coupons at TylenolProfessional.com Not a member? Register today! The efficacy and safety of TYLENOL® at 4000 mg/day are well established. Healthcare professionals may exercise their discretion and recommend up to 4000 mg. Dosage for Adults Recommended dose from your healthcare professional: mg/day TYLENOL® Regular Strength Active ingredient: acetaminophen 325 mg (per tablet) Use only as directed. DOSE & FREQUENCY DAILY LIMIT 2 tablets every 4 to 6 hours Do not take more than 10 tablets while symptoms last in 24 hours, unless directed by your doctor TYLENOL® Extra Strength Active ingredient: acetaminophen 500 mg (per caplet) Use only as directed. DOSE & FREQUENCY DAILY LIMIT 2 caplets every 6 hours while Do not take more than 6 caplets symptoms last in 24 hours, unless directed by your doctor TYLENOL® 8 HR Arthritis Pain Active ingredient: acetaminophen 650 mg (per extended-release caplet) Use only as directed. -

Benadryl Allergy Relief Plus Decongestant Capsules
Distance between cutted line/middel of the spot = 51.6 mm spot = 12 x 2 centered in the margin of 14 mm le f 14 mm GB Benadryl Allergy Relief Plus Decon Caps 12s PIL MAH Update CRD 6738 t margin 23.06.2021 English 402428175_r0 N/A 1 Brochure/Leaet/Insert 145 x 250 mm Capsules are a medicine which are used to blood pressure). 364350C IM_Val-de-Reuil_Upper Normandy_France relieve the symptoms of hay fever and similar ■ If you have glaucoma (increased pressure allergic conditions. The capsules contain in the eye). CTE LITE 364350B Westrock Saint Pierre des Corps pseudoephedrine hydrochloride, which is a ■ If you have an overactive thyroid gland. 845 Oset Printing decongestant that relieves nasal and sinus ■ If you have severe kidney problems. 7996903 PAPER congestion and acrivastine which is an ■ If you are taking, or have taken in the last EMEA_2021_00024796_003 PC-0002778 antihistamine that helps relieve allergy two weeks, drugs for depression known symptoms such as sneezing, runny nose and as Monoamine Oxidase Inhibitors CAPSULES watery eyes. (MAOIs) or Reverse Inhibitors of Acrivastine 8mg & Pseudoephedrine 60mg Monoamine Oxidase 2 Before taking this (RIMAs). ■ This medicine is used to relieve the symptoms ■ If you are taking any cough or of hay fever and similar allergic conditions. cold medicines. PANTONE PANTONE medicine 356 C 287 C ■ This medicine is for use by adults and If any of these apply to you, get advice from children aged 12 - 65 years. This medicine is suitable for most adults under LITHO OFFSET LITHO OFFSET 65 years old and children aged 12 years and a doctor or pharmacist without taking ■ Do not take this medicine: REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval. -

2019 Health for Humanity Report
2019 Health for Humanity Report Progress in Sustainability Report Summary Contents Message from Our Chairman and CEO 3 Sustainability Approach 4 2019 Year in Brief 5 Better Health for All: Tackling the World’s 6 Toughest Health Challenges Better Health for All: Access, Community 7 Health & Innovation Responsible Business Practices 8 Environmental Health 9 UNICEF, the Government of Vietnam and Johnson & Johnson are partnering on a national program to train more than 500 ethnic minority midwives in remote regions to provide effective maternal and child health interventions including early essential newborn care in village clinics and homes. Photo by Paul Bettings Front cover Volunteers, frontline health workers and government officials at the launch of the Umurinzi vaccination program in Rwanda. In October 2019, Johnson & Johnson committed to donating up to 700,000 regimens of Janssen’s investigational Ebola vaccine to support the Ebola outbreak response in Rwanda and the Democratic Republic of the Congo. Photos by Rwanda Ministry of Health 2019 Health2017 for Health Humanity for HumanityReport Summary Report 33 Message from Our Chairman and CEO Dear Johnson & Johnson Stakeholders, We know this mission will always be unfinished, and affirmed unequivocally that there is a fundamental that we will occasionally fall short. But that only serves connection between serving all stakeholders and 2019 was a year of profound change and great contrasts as motivation to move faster than we’ve ever moved generating sustainable, long-term value. around the globe. before in making bigger strides toward some of our most ambitious goals. And as we’ve detailed in this Report, we The demands for global healthcare and responsible We saw unprecedented innovation and encouraging have plenty of positive momentum worth recognizing. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

United States District Court for the Central District Of
Case 2:18-cv-09655 Document 1 Filed 11/15/18 Page 1 of 38 Page ID #:1 SIMMONS HANLY CONROY LLC 1 Crystal Foley (SBN 224627) 2 [email protected] 100 N. Sepulveda Blvd., Suite 1350 3 Los Angeles, CA 90245 4 Phone: (310) 322-3555 5 Mitchel M. Breit (pro hac vice 6 forthcoming) [email protected] 7 112 Madison Avenue 8 New York, New York 10016-7416 Phone: (212) 784-6400 9 10 Attorneys for Plaintiff 11 12 UNITED STATES DISTRICT COURT FOR THE CENTRAL DISTRICT OF CALIFORNIA 13 WESTERN DIVISION 14 TOYA EDWARDS on behalf of Case No.: ____________________2:18-cv-9655 15 herself and all others similarly 16 situated, Plaintiff, CLASS ACTION COMPLAINT 17 v. 18 DEMAND FOR JURY TRIAL WALMART, INC. 19 20 Defendant. 21 CLASS ACTION COMPLAINT 22 Plaintiff Toya Edwards individually and on behalf of all others similarly 23 situated, brings this action against Defendant Walmart, Inc. (“Walmart”). The 24 following allegations are based upon personal knowledge as to Plaintiff’s own 25 conduct, the investigation of counsel, and upon information and belief as to the acts 26 of others. 27 28 1 CLASS ACTION COMPLAINT Case 2:18-cv-09655 Document 1 Filed 11/15/18 Page 2 of 38 Page ID #:2 1 INTRODUCTION 2 1. Walmart is the world’s largest retail company operating thousands of 3 retail stores worldwide. 4 2. Walmart stores sell all types of products to the American public, 5 including toys, groceries, sports and outdoor equipment, electronics, home goods, 6 school supplies, apparel, cosmetics, health and wellness products, and many more. -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

Johnson & Johnson 1999 EPS Rose 13.8% on Sales Increase of 14.5
Johnson & Johnson 1999 EPS Rose 13.8% on Sales Increase of 14.5% Fourth Quarter EPS Rose 12.0% on Sales Increase of 6.3% NEW BRUNSWICK, N.J., Jan. 25 -- Johnson & Johnson (NYSE: JNJ - news) today announced sales for the fourth quarter of $6.9 billion, an increase of 6.3% over sales of $6.5 billion for the same period last year. Domestic sales were up 9.2% while international sales increased 2.9%. Excluding the impact of negative currency, international sales growth for the fourth quarter of 1999 was 10.2%. Worldwide sales for the year 1999 were a record $27.5 billion, an increase of 14.5% over 1998 sales of $24.0 billion. Excluding special charges, earnings per share in the fourth quarter of 1999 were $.56, an increase of 12.0% compared to $.50 for the same period in 1998. The special charges include costs associated with the Centocor merger in 1999 and the reconfiguration of the worldwide manufacturing network and in-process research and development charges in 1998. Excluding these charges, earnings per share were $2.97 for the year, compared with $2.61 per share in 1998, an increase of 13.8%. For the fourth quarter of 1999, including the special charges, the company reported earnings per share of $.53 compared with $.07 for the same period in 1998. For the year, earnings per share were $2.94, compared with $2.12 per share in 1998. Excluding special charges, consolidated net earnings for the fourth quarter were $796 million, compared with $712 million for the same period a year ago, an increase of 11.8%. -
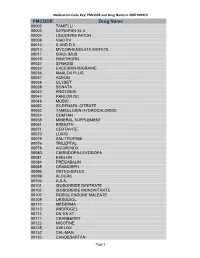
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS Cdc-Pdf
Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00002 TAMIFLU 00003 DITROPAN XL II 00004 LIDODERM PATCH 00008 VIACTIV 00010 A AND D II 00013 MYCOPHENOLATE MOFETIL 00017 SIROLIMUS 00019 HAWTHORN 00027 SYNAGIS 00032 EXCEDRIN MIGRAINE 00036 MAALOX PLUS 00037 ACEON 00038 GLYSET 00039 SONATA 00042 PROTONIX 00044 PANLOR DC 00048 MOBIC 00052 SILDENAFIL CITRATE 00053 TAMSULOSIN HYDROCHLORIDE 00054 COMTAN 00058 MINERAL SUPPLEMENT 00061 BISMUTH 00071 CERTAVITE 00073 LUXIQ 00075 SAL-TROPINE 00076 TRILEPTAL 00078 AGGRENOX 00080 CARBIDOPA-LEVODOPA 00081 EXELON 00084 PREGABALIN 00085 ORAMORPH 00096 OSTEO-BIFLEX 00099 ALOCRIL 00100 A.S.A. 00101 ISOSORBIDE DINITRATE 00102 ISOSORBIDE MONONITRATE 00107 ROSIGLITAZONE MALEATE 00109 URSODIOL 00112 MEDERMA 00113 ANDROGEL 00114 DILTIA XT 00117 CRANBERRY 00123 NICOTINE 00125 AVELOX 00132 CAL-MAG 00133 CANDESARTAN Page 1 Medication Code Key: PMCODE and Drug Name in 2007 NHHCS PMCODE Drug Name 00148 PROLIXIN D 00149 D51/2 NS 00150 NICODERM CQ PATCH 00151 TUSSIN 00152 CEREZYME 00154 CHILDREN'S IBUPROFEN 00156 PROPOXACET-N 00159 KALETRA 00161 BISOPROLOL 00167 NOVOLIN N 00169 KETOROLAC TROMETHAMINE 00172 OPHTHALMIC OINTMENT 00173 ELA-MAX 00176 PREDNISOLONE ACETATE 00179 COLLOID SILVER 00184 KEPPRA 00187 OPHTHALMIC DROPS 00190 ABDEC 00191 HAPONAL 00192 SPECTRAVITE 00198 ENOXAPARIN SODIUM 00206 ACTONEL 00208 CELECOXIB 00209 GLUCOVANCE 00211 LEVALL 5.0 00213 PANTOPRAZOLE SODIUM 00217 TEMODAR 00218 CARBAMIDE PEROXIDE 00221 CHINESE HERBAL MEDS 00224 MILK AND MOLASSES ENEMA 00238 ZOLMITRIPTAN 00239 -

View Annual Report
INVESTING FOR GROWTH Devro plc Annual Report & Accounts 2013 What’s inside this 2013 Highlights Annual Report Section 1 Strategic Report DEVRO IS ONE OF THE 2013 Highlights .................................. IFC Devro’s Business at a Glance .......... 2 WORLD’S LEADING Understanding Our Markets ........... 4 Our Strategy ............................................. 6 Key Performance Indicators ......... 14 SUPPLIERS OF COLLAGEN Chairman’s Statement ..................... 16 Chief Executive’s Review ................ 18 FOOD CASINGS Financial Review ................................ 23 Principal Risks and Uncertainties .............................. 28 Corporate Social Responsibility Report ..................... 32 Section 2 Revenue* Operating profit Report of Directors before exceptional items* Directors and Senior Management .......................... 38 £242.7m £42.1m Corporate Governance Report ... 40 Audit Committee Report ............... 47 241.1 Remuneration Report ..................... 50 242.7 227.7 42.5 42.4 42.1 Directors’ Report ............................... 64 213.6 197.4 36.2 Section 3 26.2 Financial Statements Consolidated Income Statement .............................................. 69 ‘09 ‘10 ‘11 ‘12 ‘13 ‘09 ‘10 ‘11 ‘12 ‘13 Statement of Comprehensive Income ................ 70 Balance Sheets .................................... 71 Statements of Changes in Equity .................................................. 72 Cash Flow Statements ................... 74 Earnings per share Dividends per share Notes to the Financial -

Frequently Asked Questions
Frequently Asked Questions Why are these companies included on the "Do Test" list? The following companies manufacture products that are tested on animals at some stage of development. Those marked with a Ƈ are currently observing a moratorium on (i.e., current suspension of) animal testing. Please encourage them to announce a permanent ban. Listed in parentheses are examples of products manufactured by either the company listed or, if applicable, its parent company. For a complete listing of products manufactured by a company on this list, please visit the company's website or contact the company directly for more information. Companies on this list may manufacture individual lines of products that have not been tested on animals. They have not, however, eliminated tests on animals for their entire line of cosmetics and household products. What if a company isn't on either of PETA's lists? Some companies have refused to respond to specific questions about their testing practices. It appears likely that these companies do test on animals at some stage of product development, and their refusal to clarify their testing policies appears to be an attempt to mislead consumers. Other companies may be new. If you find a company not included on our lists, please share the company's contact information with PETA so that we can contact the company directly. Legend Ƈ The company is currently observing a moratorium on animal testing. 3M 3M Corporate 1-888-364-3577 www.solutions.3m.com Headquarters 3M Center St. Paul, Minnesota 55144-1000 Acuvue (Johnson & Johnson) Customer (800) 843-2020 www.acuvue.com/ Relations, D-QA 7500 Centurion Parkway Jacksonville, Florida 32256 Aim (Church & Dwight) P.O. -

Cosmetic Formulation of Skin Care Products.Pdf
DK9685_half-series-title 4/25/06 4:34 PM Page A Cosmetic Formulation of Skin Care Products DK9685_half-series-title 4/25/06 4:34 PM Page B COSMETIC SCIENCE AND TECHNOLOGY Series Editor ERIC JUNGERMANN Jungermann Associates, Inc. Phoenix, Arizona 1. Cosmetic and Drug Preservation: Principles and Practice, edited by Jon J. Kabara 2. The Cosmetic Industry: Scientific and Regulatory Foundations, edited by Norman F. Estrin 3. Cosmetic Product Testing: A Modern Psychophysical Approach, Howard R. Moskowitz 4. Cosmetic Analysis: Selective Methods and Techniques, edited by P. Boré 5. Cosmetic Safety: A Primer for Cosmetic Scientists, edited by James H. Whittam 6. Oral Hygiene Products and Practice, Morton Pader 7. Antiperspirants and Deodorants, edited by Karl Laden and Carl B. Felger 8. Clinical Safety and Efficacy Testing of Cosmetics, edited by William C. Waggoner 9. Methods for Cutaneous Investigation, edited by Robert L. Rietschel and Thomas S. Spencer 10. Sunscreens: Development, Evaluation, and Regulatory Aspects, edited by Nicholas J. Lowe and Nadim A. Shaath 11. Glycerine: A Key Cosmetic Ingredient, edited by Eric Jungermann and Norman O. V. Sonntag 12. Handbook of Cosmetic Microbiology, Donald S. Orth 13. Rheological Properties of Cosmetics and Toiletries, edited by Dennis Laba 14. Consumer Testing and Evaluation of Personal Care Products, Howard R. Moskowitz 15. Sunscreens: Development, Evaluation, and Regulatory Aspects. Second Edition, Revised and Expanded, edited by Nicholas J. Lowe, Nadim A. Shaath, and Madhu A. Pathak DK9685_half-series-title 4/25/06 4:34 PM Page C 16. Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practice, edited by Jon J.