Electromagnetism - Lecture 10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basic Magnetic Measurement Methods
Basic magnetic measurement methods Magnetic measurements in nanoelectronics 1. Vibrating sample magnetometry and related methods 2. Magnetooptical methods 3. Other methods Introduction Magnetization is a quantity of interest in many measurements involving spintronic materials ● Biot-Savart law (1820) (Jean-Baptiste Biot (1774-1862), Félix Savart (1791-1841)) Magnetic field (the proper name is magnetic flux density [1]*) of a current carrying piece of conductor is given by: μ 0 I dl̂ ×⃗r − − ⃗ 7 1 - vacuum permeability d B= μ 0=4 π10 Hm 4 π ∣⃗r∣3 ● The unit of the magnetic flux density, Tesla (1 T=1 Wb/m2), as a derive unit of Si must be based on some measurement (force, magnetic resonance) *the alternative name is magnetic induction Introduction Magnetization is a quantity of interest in many measurements involving spintronic materials ● Biot-Savart law (1820) (Jean-Baptiste Biot (1774-1862), Félix Savart (1791-1841)) Magnetic field (the proper name is magnetic flux density [1]*) of a current carrying piece of conductor is given by: μ 0 I dl̂ ×⃗r − − ⃗ 7 1 - vacuum permeability d B= μ 0=4 π10 Hm 4 π ∣⃗r∣3 ● The Physikalisch-Technische Bundesanstalt (German national metrology institute) maintains a unit Tesla in form of coils with coil constant k (ratio of the magnetic flux density to the coil current) determined based on NMR measurements graphics from: http://www.ptb.de/cms/fileadmin/internet/fachabteilungen/abteilung_2/2.5_halbleiterphysik_und_magnetismus/2.51/realization.pdf *the alternative name is magnetic induction Introduction It -

Electrostatics Vs Magnetostatics Electrostatics Magnetostatics
Electrostatics vs Magnetostatics Electrostatics Magnetostatics Stationary charges ⇒ Constant Electric Field Steady currents ⇒ Constant Magnetic Field Coulomb’s Law Biot-Savart’s Law 1 ̂ ̂ 4 4 (Inverse Square Law) (Inverse Square Law) Electric field is the negative gradient of the Magnetic field is the curl of magnetic vector electric scalar potential. potential. 1 ′ ′ ′ ′ 4 |′| 4 |′| Electric Scalar Potential Magnetic Vector Potential Three Poisson’s equations for solving Poisson’s equation for solving electric scalar magnetic vector potential potential. Discrete 2 Physical Dipole ′′′ Continuous Magnetic Dipole Moment Electric Dipole Moment 1 1 1 3 ∙̂̂ 3 ∙̂̂ 4 4 Electric field cause by an electric dipole Magnetic field cause by a magnetic dipole Torque on an electric dipole Torque on a magnetic dipole ∙ ∙ Electric force on an electric dipole Magnetic force on a magnetic dipole ∙ ∙ Electric Potential Energy Magnetic Potential Energy of an electric dipole of a magnetic dipole Electric Dipole Moment per unit volume Magnetic Dipole Moment per unit volume (Polarisation) (Magnetisation) ∙ Volume Bound Charge Density Volume Bound Current Density ∙ Surface Bound Charge Density Surface Bound Current Density Volume Charge Density Volume Current Density Net , Free , Bound Net , Free , Bound Volume Charge Volume Current Net , Free , Bound Net ,Free , Bound 1 = Electric field = Magnetic field = Electric Displacement = Auxiliary -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Magnetism Some Basics: a Magnet Is Associated with Magnetic Lines of Force, and a North Pole and a South Pole
Materials 100A, Class 15, Magnetic Properties I Ram Seshadri MRL 2031, x6129 [email protected]; http://www.mrl.ucsb.edu/∼seshadri/teach.html Magnetism Some basics: A magnet is associated with magnetic lines of force, and a north pole and a south pole. The lines of force come out of the north pole (the source) and are pulled in to the south pole (the sink). A current in a ring or coil also produces magnetic lines of force. N S The magnetic dipole (a north-south pair) is usually represented by an arrow. Magnetic fields act on these dipoles and tend to align them. The magnetic field strength H generated by N closely spaced turns in a coil of wire carrying a current I, for a coil length of l is given by: NI H = l The units of H are amp`eres per meter (Am−1) in SI units or oersted (Oe) in CGS. 1 Am−1 = 4π × 10−3 Oe. If a coil (or solenoid) encloses a vacuum, then the magnetic flux density B generated by a field strength H from the solenoid is given by B = µ0H −7 where µ0 is the vacuum permeability. In SI units, µ0 = 4π × 10 H/m. If the solenoid encloses a medium of permeability µ (instead of the vacuum), then the magnetic flux density is given by: B = µH and µ = µrµ0 µr is the relative permeability. Materials respond to a magnetic field by developing a magnetization M which is the number of magnetic dipoles per unit volume. The magnetization is obtained from: B = µ0H + µ0M The second term, µ0M is reflective of how certain materials can actually concentrate or repel the magnetic field lines. -

Diamagnetism of Bulk Graphite Revised
magnetochemistry Article Diamagnetism of Bulk Graphite Revised Bogdan Semenenko and Pablo D. Esquinazi * Division of Superconductivity and Magnetism, Felix-Bloch-Institute for Solid State Physics, Faculty of Physics and Earth Sciences, University of Leipzig, Linnéstraße 5, 04103 Leipzig, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-341-97-32-751 Received: 15 October 2018; Accepted: 16 November 2018; Published: 22 November 2018 Abstract: Recently published structural analysis and galvanomagnetic studies of a large number of different bulk and mesoscopic graphite samples of high quality and purity reveal that the common picture assuming graphite samples as a semimetal with a homogeneous carrier density of conduction electrons is misleading. These new studies indicate that the main electrical conduction path occurs within 2D interfaces embedded in semiconducting Bernal and/or rhombohedral stacking regions. This new knowledge incites us to revise experimentally and theoretically the diamagnetism of graphite samples. We found that the c-axis susceptibility of highly pure oriented graphite samples is not really constant, but can vary several tens of percent for bulk samples with thickness t & 30 µm, whereas by a much larger factor for samples with a smaller thickness. The observed decrease of the susceptibility with sample thickness qualitatively resembles the one reported for the electrical conductivity and indicates that the main part of the c-axis diamagnetic signal is not intrinsic to the ideal graphite structure, but it is due to the highly conducting 2D interfaces. The interpretation of the main diamagnetic signal of graphite agrees with the reported description of its galvanomagnetic properties and provides a hint to understand some magnetic peculiarities of thin graphite samples. -

Module 3 : MAGNETIC FIELD Lecture 20 : Magnetism in Matter
Module 3 : MAGNETIC FIELD Lecture 20 : Magnetism in Matter Objectives In this lecture you will learn the following Study magnetic properties of matter. Express Ampere's law in the presence of magnetic matter. Define magnetization and H-vector. Understand displacement current. Assemble all the Maxwell's equations together. Study properties and propagation of electromagnetic waves in vacuum. Magnetism in Matter In our discussion on electrostatics, we have seen that in the presence of an electric field, a dielectric gets polarized, leading to bound charges. The polarization vector is, in general, in the direction of the applied electric field. A similar phenomenon occurs when a material medium is subjected to an external magnetic field. However, unlike the behaviour of dielectrics in electric field, different types of material behave in different ways when an external magnetic field is applied. We have seen that the source of magnetic field is electric current. The circulating electrons in an atom, being tiny current loops, constitute a magnetic dipole with a magnetic moment whose direction depends on the direction in which the electron is moving. An atom as a whole, may or may not have a net magnetic moment depending on the way the moments due to different elecronic orbits add up. (The situation gets further complicated because of electron spin, which is a purely quantum concept, that provides an intrinsic magnetic moment to an electron.) In the absence of a magnetic field, the atomic moments in a material are randomly oriented and consequently the net magnetic moment of the material is zero. However, in the presence of a magnetic field, the substance may acquire a net magnetic moment either in the direction of the applied field or in a direction opposite to it. -

Magnetic Susceptibility Artefact on MRI Mimicking Lymphadenopathy: Description of a Nasopharyngeal Carcinoma Patient
1161 Case Report on Focused Issue on Translational Imaging in Cancer Patient Care Magnetic susceptibility artefact on MRI mimicking lymphadenopathy: description of a nasopharyngeal carcinoma patient Feng Zhao1, Xiaokai Yu1, Jiayan Shen1, Xinke Li1, Guorong Yao1, Xiaoli Sun1, Fang Wang1, Hua Zhou2, Zhongjie Lu1, Senxiang Yan1 1Department of Radiation Oncology, 2Department of Radiology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, China Correspondence to: Zhongjie Lu. Department of Radiation Oncology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, China. Email: [email protected]; Senxiang Yan. Department of Radiation Oncology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, China. Email: [email protected]. Abstract: This report describes a case involving a 34-year-old male patient with nasopharyngeal carcinoma (NPC) who exhibited the manifestation of lymph node enlargement on magnetic resonance (MR) images due to a magnetic susceptibility artefact (MSA). Initial axial T1-weighted and T2-weighted images acquired before treatment showed a round nodule with hyperintensity in the left retropharyngeal space that mimicked an enlarged cervical lymph node. However, coronal T2-weighted images and subsequent computed tomography (CT) images indicated that this lymph node-like lesion was an MSA caused by the air-bone tissue interface rather than an actual lymph node or another artefact. In addition, for the subsequent MR imaging (MRI), which was performed after chemoradiotherapy (CRT) treatment for NPC, axial MR images also showed an enlarged lymph node-like lesion. This MSA was not observed in a follow-up MRI examination when different MR sequences were used. -

Magnetism in Transition Metal Complexes
Magnetism for Chemists I. Introduction to Magnetism II. Survey of Magnetic Behavior III. Van Vleck’s Equation III. Applications A. Complexed ions and SOC B. Inter-Atomic Magnetic “Exchange” Interactions © 2012, K.S. Suslick Magnetism Intro 1. Magnetic properties depend on # of unpaired e- and how they interact with one another. 2. Magnetic susceptibility measures ease of alignment of electron spins in an external magnetic field . 3. Magnetic response of e- to an external magnetic field ~ 1000 times that of even the most magnetic nuclei. 4. Best definition of a magnet: a solid in which more electrons point in one direction than in any other direction © 2012, K.S. Suslick 1 Uses of Magnetic Susceptibility 1. Determine # of unpaired e- 2. Magnitude of Spin-Orbit Coupling. 3. Thermal populations of low lying excited states (e.g., spin-crossover complexes). 4. Intra- and Inter- Molecular magnetic exchange interactions. © 2012, K.S. Suslick Response to a Magnetic Field • For a given Hexternal, the magnetic field in the material is B B = Magnetic Induction (tesla) inside the material current I • Magnetic susceptibility, (dimensionless) B > 0 measures the vacuum = 0 material response < 0 relative to a vacuum. H © 2012, K.S. Suslick 2 Magnetic field definitions B – magnetic induction Two quantities H – magnetic intensity describing a magnetic field (Système Internationale, SI) In vacuum: B = µ0H -7 -2 µ0 = 4π · 10 N A - the permeability of free space (the permeability constant) B = H (cgs: centimeter, gram, second) © 2012, K.S. Suslick Magnetism: Definitions The magnetic field inside a substance differs from the free- space value of the applied field: → → → H = H0 + ∆H inside sample applied field shielding/deshielding due to induced internal field Usually, this equation is rewritten as (physicists use B for H): → → → B = H0 + 4 π M magnetic induction magnetization (mag. -
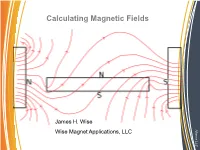
Calculating Magnetic Fields, Please Call
Calculating Magnetic Fields James H. Wise Alliance LLC Alliance Wise Magnet Applications, LLC Magnetic Fields: What and Where? Magnetic fields: Sources: • Appear around electric currents • Surround magnetic materials Properties: • They have a direction • They possess magnitude • They are vector fields Alliance LLC Alliance Reference for Definition Field descriptions and behavior See Chapters 1, 2 and 3 of “The Feynman Lectures in Physics”, Vol.II by Feynman, Leighton and Sands, Addison Wesley,1964, ISBN 0 -201-2117-X-P . Chapter 1 is my favorite starting point for thinking about fields. The next slide is derived from that. Alliance LLC Alliance Examples of Fields Fields: • When a quantity varies with location in space, (x,y,z,t), it is said to be a field. • Temperature around a heat source forms a field. • As the temperature is only a single quantity associated with it is said to be a scalar field • Heat flow has a direction and thus 3 quantities that change with (x,y,z) and perhaps a time dependence • Because it has 3 spatial components obeying the rules of vectors, it is said to be a vector field. Alliance LLC Alliance Magnetic Fields of Permanent Magnets • The magnetic field was originally treated as originating from charges. • Pole strength was interpreted as the amount of magnetic charge on the surface of a magnet If the charge distribution was known, the force outside the magnet could be computed correctly • The charge model does not give the correct results for fields inside magnets. • The pole model is useful for gaining an intuitive view for qualitative results. -

Electromagnetism 3. Magnetization (Lectures 7–9)
Electromagnetism Handout 3: 3. Magnetization (Lectures 7{9): Here is the proof of a useful vector identity. First recall Stokes' theorem which states that Z I r × A · dS = A · dl: (1) Let us suppose that A takes the form A(r) = f(r)c where c 6= c(r) is a constant vector. Hence we have r × A = fr × c − c × rf = −c × rf (2) since r × c = 0 (because c is a constant vector, its derivative vanishes). Stokes' theorem then gives us I I Z Z A · dl = c · f dl = − c × rf · dS = −c · rf × dS; (3) but this is true for any c and so I Z f dl = − rf × dS: (4) Now let us choose f = r^ · r0 and take the gradient and integral around a closed loop in the primed coordinates: I Z Z (r^ · r0) dl0 = − r0(r^ · r0) × dS0 = −r^ × dS0 (5) which works because r0(r^ · r0) = r^. In summary: I Z r^ · r0 dl0 = dS0 × r^: (6) We will use this to show that the magnetic vector potential from a magnetic dipole is given by µ A = 0 m × r^; (7) 4πr2 where m is the magnetic dipole moment. For example, if we put m parallel to z and use spherical polars, we have that µ A = 0 m sin θ φ^; (8) 4πr2 and so 1 @ 1 @ B = r × A(r) = (r sin θ A )r^ − (r sin θ A )θ^; (9) r2 sin θ @θ φ r sin θ @r φ because Ar = Aθ = 0 and hence µ m 2 cos θ sin θ B = 0 r^ + θ^ ; (10) 4π r3 r3 which is the same form as an electric dipole. -

Condensed Matter Option MAGNETISM Handout 1
Condensed Matter Option MAGNETISM Handout 1 Hilary 2014 Radu Coldea http://www2.physics.ox.ac.uk/students/course-materials/c3-condensed-matter-major-option Syllabus The lecture course on Magnetism in Condensed Matter Physics will be given in 7 lectures broken up into three parts as follows: 1. Isolated Ions Magnetic properties become particularly simple if we are able to ignore the interactions between ions. In this case we are able to treat the ions as effectively \isolated" and can discuss diamagnetism and paramagnetism. For the latter phenomenon we revise the derivation of the Brillouin function outlined in the third-year course. Ions in a solid interact with the crystal field and this strongly affects their properties, which can be probed experimentally using magnetic resonance (in particular ESR and NMR). 2. Interactions Now we turn on the interactions! I will discuss what sort of magnetic interactions there might be, including dipolar interactions and the different types of exchange interaction. The interactions lead to various types of ordered magnetic structures which can be measured using neutron diffraction. I will then discuss the mean-field Weiss model of ferromagnetism, antiferromagnetism and ferrimagnetism and also consider the magnetism of metals. 3. Symmetry breaking The concept of broken symmetry is at the heart of condensed matter physics. These lectures aim to explain how the existence of the crystalline order in solids, ferromagnetism and ferroelectricity, are all the result of symmetry breaking. The consequences of breaking symmetry are that systems show some kind of rigidity (in the case of ferromagnetism this is permanent magnetism), low temperature elementary excitations (in the case of ferromagnetism these are spin waves, also known as magnons), and defects (in the case of ferromagnetism these are domain walls). -

Magnetic Susceptibility Artifacts in Gradient-Recalled Echo MR Imaging
1149 Magnetic Susceptibility Artifacts in Gradient-Recalled Echo MR Imaging Leo F. Czervionke1 Artifacts related to magnetic susceptibility differences between bone and soft tissue David L. Daniels 1 are prevalent on gradient-recalled echo images, particularly when long echo delay times Felix W . Wehrli2 are used. These susceptibility artifacts spatially distort and artifactually enlarge bone Leighton P. Mark1 contours. This can alter the apparent shape of the spinal canal and exaggerate the Lloyd E. Hendrix1 degree of spinal stenosis seen in patients with cervical spondylosis. The effects of magnetic susceptibility artifacts in gradient echo imaging were studied Julie A. Strandt1 1 in a phantom model and the results were correlated with MR images obtained in patients Alan L. Williams with cervical spondylosis. Victor M. Haughton1 Magnetic susceptibility is the ratio of the intensity of magnetization produced in a substance to the intensity of the applied magnetic field [1]. In other words, magnetic susceptibility is a measure of the extent a substance can be magnetized when placed in an external magnetic field . In spin echo imaging, ferromagnetic materials have high magnetic susceptibility, which alters the homogeneity of the field and results in geometric distortion of the image [2] . To a lesser extent such geometric distortion has been observed near air/tissue interfaces when gradient recalled echoes (GRE) are used [3]. Spatial variations in magnetic or diamagnetic susceptibility cause intrinsic magnetic field gradients across the imaging voxel. Spins subjected to these gradients lose phase coherence with a concomitant attenuation in signal intensity, leading to the well-known artifactual loss of signal intensity near air-containing sinus cavities.