Introduction to Radiation Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiant Heating with Infrared
W A T L O W RADIANT HEATING WITH INFRARED A TECHNICAL GUIDE TO UNDERSTANDING AND APPLYING INFRARED HEATERS Bleed Contents Topic Page The Advantages of Radiant Heat . 1 The Theory of Radiant Heat Transfer . 2 Problem Solving . 14 Controlling Radiant Heaters . 25 Tips On Oven Design . 29 Watlow RAYMAX® Heater Specifications . 34 The purpose of this technical guide is to assist customers in their oven design process, not to put Watlow in the position of designing (and guaranteeing) radiant ovens. The final responsibility for an oven design must remain with the equipment builder. This technical guide will provide you with an understanding of infrared radiant heating theory and application principles. It also contains examples and formulas used in determining specifications for a radiant heating application. To further understand electric heating principles, thermal system dynamics, infrared temperature sensing, temperature control and power control, the following information is also available from Watlow: • Watlow Product Catalog • Watlow Application Guide • Watlow Infrared Technical Guide to Understanding and Applying Infrared Temperature Sensors • Infrared Technical Letter #5-Emissivity Table • Radiant Technical Letter #11-Energy Uniformity of a Radiant Panel © Watlow Electric Manufacturing Company, 1997 The Advantages of Radiant Heat Electric radiant heat has many benefits over the alternative heating methods of conduction and convection: • Non-Contact Heating Radiant heaters have the ability to heat a product without physically contacting it. This can be advantageous when the product must be heated while in motion or when physical contact would contaminate or mar the product’s surface finish. • Fast Response Low thermal inertia of an infrared radiation heating system eliminates the need for long pre-heat cycles. -

A Compilation of Data on the Radiant Emissivity of Some Materials at High Temperatures
This is a repository copy of A compilation of data on the radiant emissivity of some materials at high temperatures. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/133266/ Version: Accepted Version Article: Jones, JM orcid.org/0000-0001-8687-9869, Mason, PE and Williams, A (2019) A compilation of data on the radiant emissivity of some materials at high temperatures. Journal of the Energy Institute, 92 (3). pp. 523-534. ISSN 1743-9671 https://doi.org/10.1016/j.joei.2018.04.006 © 2018 Energy Institute. Published by Elsevier Ltd. This is an author produced version of a paper published in Journal of the Energy Institute. Uploaded in accordance with the publisher's self-archiving policy. This manuscript version is made available under the Creative Commons CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ A COMPILATION OF DATA ON THE RADIANT EMISSIVITY OF SOME MATERIALS AT HIGH TEMPERATURES J.M Jones, P E Mason and A. -
Plant Lighting Basics and Applications
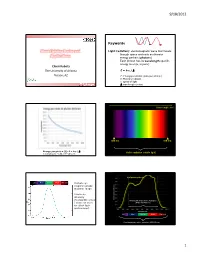
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Spectral Reflectance and Emissivity of Man-Made Surfaces Contaminated with Environmental Effects
Optical Engineering 47͑10͒, 106201 ͑October 2008͒ Spectral reflectance and emissivity of man-made surfaces contaminated with environmental effects John P. Kerekes, MEMBER SPIE Abstract. Spectral remote sensing has evolved considerably from the Rochester Institute of Technology early days of airborne scanners of the 1960’s and the first Landsat mul- Chester F. Carlson Center for Imaging Science tispectral satellite sensors of the 1970’s. Today, airborne and satellite 54 Lomb Memorial Drive hyperspectral sensors provide images in hundreds of contiguous narrow Rochester, New York 14623 spectral channels at spatial resolutions down to meter scale and span- E-mail: [email protected] ning the optical spectral range of 0.4 to 14 m. Spectral reflectance and emissivity databases find use not only in interpreting these images but also during simulation and modeling efforts. However, nearly all existing Kristin-Elke Strackerjan databases have measurements of materials under pristine conditions. Aerospace Engineering Test Establishment The work presented extends these measurements to nonpristine condi- P.O. Box 6550 Station Forces tions, including materials contaminated with sand and rain water. In par- Cold Lake, Alberta ticular, high resolution spectral reflectance and emissivity curves are pre- T9M 2C6 Canada sented for several man-made surfaces ͑asphalt, concrete, roofing shingles, and vehicles͒ under varying amounts of sand and water. The relationship between reflectance and area coverage of the contaminant Carl Salvaggio, MEMBER SPIE is reported and found to be linear or nonlinear, depending on the mate- Rochester Institute of Technology rials and spectral region. In addition, new measurement techniques are Chester F. Carlson Center for Imaging Science presented that overcome limitations of existing instrumentation and labo- 54 Lomb Memorial Drive ratory settings. -

TIE-35: Transmittance of Optical Glass
DATE October 2005 . PAGE 1/12 TIE-35: Transmittance of optical glass 0. Introduction Optical glasses are optimized to provide excellent transmittance throughout the total visible range from 400 to 800 nm. Usually the transmittance range spreads also into the near UV and IR regions. As a general trend lowest refractive index glasses show high transmittance far down to short wavelengths in the UV. Going to higher index glasses the UV absorption edge moves closer to the visible range. For highest index glass and larger thickness the absorption edge already reaches into the visible range. This UV-edge shift with increasing refractive index is explained by the general theory of absorbing dielectric media. So it may not be overcome in general. However, due to improved melting technology high refractive index glasses are offered nowadays with better blue-violet transmittance than in the past. And for special applications where best transmittance is required SCHOTT offers improved quality grades like for SF57 the grade SF57 HHT. The aim of this technical information is to give the optical designer a deeper understanding on the transmittance properties of optical glass. 1. Theoretical background A light beam with the intensity I0 falls onto a glass plate having a thickness d (figure 1-1). At the entrance surface part of the beam is reflected. Therefore after that the intensity of the beam is Ii0=I0(1-r) with r being the reflectivity. Inside the glass the light beam is attenuated according to the exponential function. At the exit surface the beam intensity is I i = I i0 exp(-kd) [1.1] where k is the absorption constant. -

Reflectance and Transmittance Sphere
Reflectance and Transmittance Sphere Spectral Evolution four inch portable reflectance/ transmittance sphere is ideal for measuring vegetation physiological states SPECTRAL EVOLUTION offers a portable, compact four inch reflectance/transmittance (R/T) integrating sphere for measuring the reflectance and transmittance of vegetation. The 4 inch R/T sphere is lightweight and portable so you can take it into the field for in situ meas- urements, delivered with a stand as well as a ¼-20 mount for use with tripods. When used with a SPECTRAL EVOLUTION spectrometer or spectroradiometer such as the PSR+, RS-3500, RS- 5400, SR-6500 or RS-8800, it delivers detailed information for measurements of transmittance and reflectance modes. Two varying light intensity levels (High and Low) allow for a broader range of samples to be measured. Light reflected or transmitted from a sample in a sphere is integrated over a full hemisphere, with measurements insensitive to sample anisotropic directional reflectance (transmittance). This provides repeatable measurements of a variety of samples. The 4 inch portable R/T sphere can be used in vegetation studies such as, deriving absorbance characteristics, and estimating the leaf area index (LAI) from radiation reflected from a canopy surface. The sphere allows researchers to limit destructive sampling and provides timely data acquisition of key material samples. The R/T sphere allows you to collect all diffuse light reflected from a sample – measuring total hemispherical reflectance. You can measure reflectance and transmittance, and calculate absorption. You can select the bulb inten- sity level that suits your sample. A white reference is provided for both reflectance and transmittance measurements. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

Black Body Radiation and Radiometric Parameters
Black Body Radiation and Radiometric Parameters: All materials absorb and emit radiation to some extent. A blackbody is an idealization of how materials emit and absorb radiation. It can be used as a reference for real source properties. An ideal blackbody absorbs all incident radiation and does not reflect. This is true at all wavelengths and angles of incidence. Thermodynamic principals dictates that the BB must also radiate at all ’s and angles. The basic properties of a BB can be summarized as: 1. Perfect absorber/emitter at all ’s and angles of emission/incidence. Cavity BB 2. The total radiant energy emitted is only a function of the BB temperature. 3. Emits the maximum possible radiant energy from a body at a given temperature. 4. The BB radiation field does not depend on the shape of the cavity. The radiation field must be homogeneous and isotropic. T If the radiation going from a BB of one shape to another (both at the same T) were different it would cause a cooling or heating of one or the other cavity. This would violate the 1st Law of Thermodynamics. T T A B Radiometric Parameters: 1. Solid Angle dA d r 2 where dA is the surface area of a segment of a sphere surrounding a point. r d A r is the distance from the point on the source to the sphere. The solid angle looks like a cone with a spherical cap. z r d r r sind y r sin x An element of area of a sphere 2 dA rsin d d Therefore dd sin d The full solid angle surrounding a point source is: 2 dd sind 00 2cos 0 4 Or integrating to other angles < : 21cos The unit of solid angle is steradian. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
Properties of Electromagnetic Waves Any Electromagnetic Wave Must Satisfy Four Basic Conditions: 1
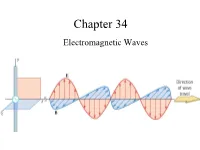
Chapter 34 Electromagnetic Waves The Goal of the Entire Course Maxwell’s Equations: Maxwell’s Equations James Clerk Maxwell •1831 – 1879 •Scottish theoretical physicist •Developed the electromagnetic theory of light •His successful interpretation of the electromagnetic field resulted in the field equations that bear his name. •Also developed and explained – Kinetic theory of gases – Nature of Saturn’s rings – Color vision Start at 12:50 https://www.learner.org/vod/vod_window.html?pid=604 Correcting Ampere’s Law Two surfaces S1 and S2 near the plate of a capacitor are bounded by the same path P. Ampere’s Law states that But it is zero on S2 since there is no conduction current through it. This is a contradiction. Maxwell fixed it by introducing the displacement current: Fig. 34-1, p. 984 Maxwell hypothesized that a changing electric field creates an induced magnetic field. Induced Fields . An increasing solenoid current causes an increasing magnetic field, which induces a circular electric field. An increasing capacitor charge causes an increasing electric field, which induces a circular magnetic field. Slide 34-50 Displacement Current d d(EA)d(q / ε) 1 dq E 0 dt dt dt ε0 dt dq d ε E dt0 dt The displacement current is equal to the conduction current!!! Bsd μ I μ ε I o o o d Maxwell’s Equations The First Unified Field Theory In his unified theory of electromagnetism, Maxwell showed that electromagnetic waves are a natural consequence of the fundamental laws expressed in these four equations: q EABAdd 0 εo dd Edd s BE B s μ I μ ε dto o o dt QuickCheck 34.4 The electric field is increasing.