An Improved Model of Carbon and Nutrient Dynamics in the Microbial Food Web in Marine Enclosures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Destabilization, Stabilization, and Multiple Attractors in Saturated Mixotrophic Environments
Downloaded from orbit.dtu.dk on: Sep 27, 2021 Destabilization, stabilization, and multiple attractors in saturated mixotrophic environments Lindstrom, Torsten; Cheng, Yuanji; Chakraborty, Subhendu Published in: SIAM Journal on Applied Mathematics Link to article, DOI: 10.1137/19M1294186 Publication date: 2020 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Lindstrom, T., Cheng, Y., & Chakraborty, S. (2020). Destabilization, stabilization, and multiple attractors in saturated mixotrophic environments. SIAM Journal on Applied Mathematics, 80(6), 2338-2364. https://doi.org/10.1137/19M1294186 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. SIAM J. APPL.MATH. © 2020 Society for Industrial and Applied Mathematics Vol. 80, No. 6, pp. 2338{2364 DESTABILIZATION, STABILIZATION, AND MULTIPLE ATTRACTORS IN SATURATED MIXOTROPHIC ENVIRONMENTS\ast \" y z x TORSTEN LINDSTROM , YUANJI CHENG , AND SUBHENDU CHAKRABORTY Abstract. The ability of mixotrophs to combine phototrophy and phagotrophy is now well recognized and found to have important implications for ecosystem dynamics. -

Bioenergetics of Mixotrophic Metabolisms: a Theoretical Analysis
Bioenergetics of mixotrophic metabolisms: A theoretical analysis by Stephanie Slowinski A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Science in Earth Sciences (Water) Waterloo, Ontario, Canada, 2019 © Stephanie Slowinski 2019 Author’s Declaration This thesis consists of material all of which I authored or co-authored: see Statement of Contributions included in the thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Statement of Contributions This thesis consists of two co-authored chapters. I contributed to the study design and execution in chapters 2 and 3. Christina M. Smeaton (CMS) and Philippe Van Cappellen (PVC) provided guidance during the study design and analysis. I co-authored both of these chapters with CMS and PVC. iii Abstract Many biogeochemical reactions controlling surface water and groundwater quality, as well as greenhouse gas emissions and carbon turnover rates, are catalyzed by microorganisms. Representing the thermodynamic (or bioenergetic) constraints on the reduction-oxidation reactions carried out by microorganisms in the subsurface is essential to understand and predict how microbial activity affects the environmental fate and transport of chemicals. While organic compounds are often considered to be the primary electron donors (EDs) in the subsurface, many microorganisms use inorganic EDs and are capable of autotrophic carbon fixation. Furthermore, many microorganisms and communities are likely capable of mixotrophy, switching between heterotrophic and autotrophic metabolisms according to the environmental conditions and energetic substrates available to them. -

MIXOTROPHY in FRESHWATER FOOD WEBS a Dissertation
MIXOTROPHY IN FRESHWATER FOOD WEBS A Dissertation Submitted to the Temple University Graduate Board In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY by Sarah B. DeVaul May 2016 Examining Committee Members: Robert W. Sanders, Advisory Chair, Biology Erik E. Cordes, Biology Amy Freestone, Biology Dale Holen, External Member, Penn State Worthington Scranton © Copyright 2016 by Sarah B. DeVaul All Rights Reserved ii ABSTRACT Environmental heterogeneity in both space and time has significant repercussions for community structure and ecosystem processes. Dimictic lakes provide examples of vertically structured ecosystems that oscillate between stable and mixed thermal layers on a seasonal basis. Vertical patterns in abiotic conditions vary during both states, but with differing degrees of variation. For example, during summer thermal stratification there is high spatial heterogeneity in temperature, nutrients, dissolved oxygen and photosynthetically active radiation. The breakdown of stratification and subsequent mixing of the water column in fall greatly reduces the stability of the water column to a vertical gradient in light. Nutrients and biomass that were otherwise constrained to the depths are also suspended, leading to a boom in productivity. Freshwater lakes are teeming with microbial diversity that responds to the dynamic environment in a seemingly predictable manner. Although such patterns have been well studied for nanoplanktonic phototrophic and heterotrophic populations, less work has been done to integrate the influence of mixotrophic nutrition to the protistan assemblage. Phagotrophy by phytoplankton increases the complexity of nutrient and energy flow due to their dual functioning as producers and consumers. The role of mixotrophs in freshwater planktonic communities also varies depending on the relative balance between taxon-specific utilization of carbon and energy sources that ranges widely between phototrophy and heterotrophy. -

Mixotrophy in Planktonic Communities Title: Intraguild Predation Enables
Running Head: Mixotrophy in planktonic communities Title: Intraguild predation enables coexistence of competing phytoplankton in a well-mixed water column Authors: Holly V. Moeller1,2,3,∗, Michael G. Neubert1, and Matthew D. Johnson1 1. Biology Department, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts 02543, USA; 2. Biodiversity Research Centre, University of British Columbia, Vancouver, British Columbia V6T 1Z4, Canada. 3. Current Address: Department of Ecology, Evolution, & Marine Biology, University of Califor- nia, Santa Barbara, Santa Barbara, California 93106, USA; ∗ Corresponding author; email: [email protected] Manuscript elements: 5 figures, 1 table, online appendix A (including figures A1-9). Figures 1, 4, 5, and A1-A3 are in color. Manuscript type: Article 1 Abstract: Resource competition theory predicts that when two species compete for a single, finite resource, 3 the better competitor should exclude the other. However, in some cases, weaker competitors can persist through intraguild predation, i.e., by eating their stronger competitor. Mixotrophs, species that meet their carbon demand by combining photosynthesis and phagotrophic heterotro- 6 phy, may function as intraguild predators when they consume the phototrophs with which they compete for light. Thus, theory predicts that mixotrophy may allow for coexistence of two species on a single limiting resource. We tested this prediction by developing a new mathematical model 9 for a unicellular mixotroph and phytoplankter that compete for light, and comparing the model’s predictions with a laboratory experimental system. We find that, like other intraguild predators, mixotrophs can persist when an ecosystem is sufficiently productive (i.e., the supply of the lim- 12 iting resource, light, is relatively high), or when species interactions are strong (i.e., attack rates and conversion efficiencies are high). -

Microbial Sulfur Transformations in Sediments from Subglacial Lake Whillans
ORIGINAL RESEARCH ARTICLE published: 19 November 2014 doi: 10.3389/fmicb.2014.00594 Microbial sulfur transformations in sediments from Subglacial Lake Whillans Alicia M. Purcell 1, Jill A. Mikucki 1*, Amanda M. Achberger 2 , Irina A. Alekhina 3 , Carlo Barbante 4 , Brent C. Christner 2 , Dhritiman Ghosh1, Alexander B. Michaud 5 , Andrew C. Mitchell 6 , John C. Priscu 5 , Reed Scherer 7 , Mark L. Skidmore8 , Trista J. Vick-Majors 5 and the WISSARD Science Team 1 Department of Microbiology, University of Tennessee, Knoxville, TN, USA 2 Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA 3 Climate and Environmental Research Laboratory, Arctic and Antarctic Research Institute, St. Petersburg, Russia 4 Institute for the Dynamics of Environmental Processes – Consiglio Nazionale delle Ricerche and Department of Environmental Sciences, Informatics and Statistics, Ca’ Foscari University of Venice, Venice, Italy 5 Department of Land Resources and Environmental Sciences, Montana State University, Bozeman, MT, USA 6 Geography and Earth Sciences, Aberystwyth University, Ceredigion, UK 7 Department of Geological and Environmental Sciences, Northern Illinois University, DeKalb, IL, USA 8 Department of Earth Sciences, Montana State University, Bozeman, MT, USA Edited by: Diverse microbial assemblages inhabit subglacial aquatic environments.While few of these Andreas Teske, University of North environments have been sampled, data reveal that subglacial organisms gain energy for Carolina at Chapel Hill, USA growth from reduced minerals containing nitrogen, iron, and sulfur. Here we investigate Reviewed by: the role of microbially mediated sulfur transformations in sediments from Subglacial Aharon Oren, The Hebrew University of Jerusalem, Israel Lake Whillans (SLW), Antarctica, by examining key genes involved in dissimilatory sulfur John B. -

Explorations of Aspects of Mixotrophy Using
EXPLORATIONS OF ASPECTS OF MIXOTROPHY USING MATHEMATICAL MODELS by KENNETH W. CRANE Presented to the Faculty of the Graduate School of The University of Texas at Arlington in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY THE UNIVERSITY OF TEXAS AT ARLINGTON August 2010 Copyright © by Kenneth W. Crane 2010 All rights reserved ACKNOWLEDGEMENTS This work could not have been completed without the financial support of the Biology Department and the University in the form of teaching assistantships and fellowships. I especially want to thank the Graduate School for providing the Graduate Dean’s Dissertation Fellowship that made the completion of this dissertation possible. My supervising professor, James P. Grover, made the completion of this work possible with advice on academics, research topics and methodologies, writing, and the funding of a research assistantship. I would also like to thank the other members of my committee, Thomas Chrzanowski, Laura Gough, Hristo Kojuharov, and Laura Mydalrz, for their time and advice. Finally, the financial and moral support of my family was essential to the completion of this challenge. July 8, 2010 iii ABSTRACT EXPLORATIONS OF ASPECTS OF MIXOTROPHY USING MATHEMATICAL MODELS Kenneth W. Crane, Ph.D. The University of Texas at Arlington, 2010 Supervising Professor: James P. Grover This work examined three theoretical aspects of mixotrophy, the simultaneous use of phototrophic and heterotrophic nutritional modes, and one practical application. The first study addressed the coexistence of microorganisms pursuing diverse nutritional strategies. It was found that in the range of model environments explored that all four types of organisms were predicted to persist for certain parameter combinations. -

Reasons to Sequence the Genome of the Dinoflagellate Symbiodinium Thomas G
Symbiodinum_WPl+Appendix_v2.doc 1 Reasons to sequence the genome of the dinoflagellate Symbiodinium Thomas G. Doak, Princeton, USA. <[email protected]> Robert B. Moore, University of Sydney, AU. <[email protected]> Charles Delwiche University of Maryland at College Park, USA <[email protected]> Ove Hoegh-Guldberg, University of Queensland, AU. <[email protected]> Mary Alice Coffroth, State University of New York at Buffalo, USA. <[email protected]> Abstract Dinoflagellates are ubiquitous marine and freshwater protists. As free-living photosynthetic plankton, they account for much of the primary productivity of oceans and lakes. As photosynthetic symbionts, they provide essential nutrients to all reef-building corals and numerous other marine invertebrates, supporting coral reefs, the most diverse marine ecosystem, a rich food source, and a potential source of future pharmaceuticals. When the dinoflagellate symbionts are expelled from their coral hosts during mass coral bleaching events, coral reefs and the surrounding ecosystems rapidly decline and die. Population explosions of dinoflagellates are the cause of red tides, extensive fish kills, and paralytic shellfish poisoning. As parasites and predators, they threaten numerous fisheries, but are also an important check on the very dinoflagellates that cause red tides. As bioluminescent organisms, they are a spectacular feature of nighttime oceans and a model for the study of luminescence chemistry. Dinoflagellates are alveolates and a sister group to the apicomplexans–important human and animal pathogens. The apicomplexans are obligate intracellular parasites, e.g. Plasmodium spp. the agents of malaria. Describing the genome of a dinoflagellate that is an intracellular symbiont will aid us in understanding the capacities, weaknesses and evolution of the apicomplexans, and will inform the apicomplexan genomes in a way directly relevant to the development of antimalarial and anti- apicomplexan drugs. -

Ecophysiology of Mixotrophs
Ecophysiology of mixotrophs Erwin Schoonhoven January 19, 2000 Contents 1 Introduction 3 1.1 Definition of the mixotroph . 3 2 Different strategies in mixotrophy 6 3 Mixotrophic organisms and their ways of mixotrophic feeding 8 3.1 Mixotrophic organisms . 8 3.1.1 Phytoplankton . 8 3.1.2 Planktonic ciliates . 8 3.1.3 Cyanobacteria . 9 3.1.4 Zooplankton, multicellular organisms . 9 3.2 Mixotrophic feeding . 9 3.2.1 Obligate mixotrophs . 9 3.2.2 Facultative mixotrophs . 10 3.2.3 `True' facultative mixotrophs . 14 4 Basics of feeding 16 4.1 Usage of both strategies in a obligate mixotroph . 16 4.2 Switching in a facultative mixotroph . 16 4.2.1 Facultative mixotroph that is obligate phototrophic . 17 4.2.2 Facultative mixotroph that is obligate heterotrophic . 17 4.3 Switching in the `true' facultative mixotroph . 18 5 Conceptual dynamics of feeding 20 5.1 Usage of both strategies in the obligate mixotroph . 20 5.2 Switching in the facultative mixotroph . 21 5.2.1 Facultative mixotroph that is obligate phototrophic . 22 5.2.2 Facultative mixotroph that is obligate heterotrophic . 23 5.3 Dynamics of feeding in the `true' facultative mixotroph . 24 6 Models for mixotrophy 27 6.0.1 A list of data on the ecophysiology of mixotrophs . 28 7 Symbiosis and mixotrophy, the same thing? 29 8 Conclusions 31 2 Chapter 1 Introduction 1.1 Definition of the mixotroph Mixotrophy is defined as the capability of one organism to be autotrophic and heterotrophic at the same time. Therefor an organism that can survive in water as a chemolitho-autotroph and, at the same time, is capable of ingestion and digestion of organic compounds from human blood, is called a mixotroph. -

Harmful Algal Blooms and Their Effects in Coastal Seas of Northern Europe
Harmful Algae xxx (xxxx) xxx Contents lists available at ScienceDirect Harmful Algae journal homepage: www.elsevier.com/locate/hal Review Harmful algal blooms and their effects in coastal seas of Northern Europe Bengt Karlson a,*, Per Andersen b, Lars Arneborg a, Allan Cembella c, Wenche Eikrem d,e, Uwe John c,f, Jennifer Joy West g, Kerstin Klemm c, Justyna Kobos h, Sirpa Lehtinen i, Nina Lundholm j, Hanna Mazur-Marzec h, Lars Naustvoll k, Marnix Poelman l, Pieter Provoost m, Maarten De Rijcke n, Sanna Suikkanen i a Swedish Meteorological and Hydrological Institute, Research and Development, Oceanography, Sven Kallfelts¨ gata 15, SE-426 71 Vastra¨ Frolunda,¨ Sweden b Aarhus University, Marine Ecology, Vejlsøvej 25, 8600 Silkeborg, Denmark c Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Am Handelshafen 12, 27570 Bremerhaven, Germany d University of Oslo, Department of Biosciences, P. O. Box 1066 Blindern, Oslo 0316, Norway e Norwegian Institute for Water Research. Gaustadalleen 21, 0349 Oslo, Norway f Helmholtz Institute for Functional Marine Biodiversity, Ammerlander¨ Heerstraße 231, 26129 Oldenburg, Germany g CICERO Center for International Climate Research, P.O. Box 1129, 0318 Blindern, Oslo Norway h University of Gdansk, Institute of Oceanography, Division of Marine Biotechnology, Marszalka Pilsudskiego 46, 81-378 Gdynia; POLAND i Finnish Environment Institute (SYKE), Marine Research Centre, Agnes Sjobergin¨ katu 2, 00790 Helsinki, Finland j Natural History Museum of Denmark, University of Copenhagen, Øster -

Mixotrophy in Nanoflagellates Across Environmental Gradients in the Ocean
Mixotrophy in nanoflagellates across environmental SEE COMMENTARY gradients in the ocean Kyle F. Edwardsa,1 aDepartment of Oceanography, University of Hawai‘iatManoa, Honolulu, HI 96822 Edited by David M. Karl, University of Hawai‘iatManoa, Honolulu, HI, and approved January 18, 2019 (received for review August 28, 2018) Mixotrophy, the combination of autotrophic and heterotrophic that constrain mixotrophic performance, and food web structure. nutrition, is a common trophic strategy among unicellular eukaryotes For example, Ward et al. (11) assumed that nutrient uptake and in the ocean. There are a number of hypotheses about the conditions phagotrophy are mutually limited by cell surface area and found that select for mixotrophy, and field studies have documented the that mixotrophs competing with autotrophs and heterotrophs prevalence of mixotrophy in a range of environments. However, can persist only when nutrient uptake and prey capture are there is currently little evidence for how mixotrophy varies across diffusion-limited, as opposed to transport-limited. Irradiance environmental gradients, and whether empirical patterns support may also play a key role in the prevalence of mixotrophy, because theoretical predictions. Here I synthesize experiments that have with ample carbon obtained through photosynthesis (relative to quantified the abundance of phototrophic, mixotrophic, and hetero- grazing) a mixotroph may suppress prey density below a spe- trophic nanoflagellates, to ask whether there are broad patterns in cialist heterotroph’s requirements (12). This prediction has been the prevalence of mixotrophy (relative to pure autotrophy and supported by laboratory competition experiments (10, 12, 13) heterotrophy), and to ask whether observed patterns are consistent and may explain why trophic strategies vary with depth in lakes with a trait-based model of trophic strategies. -

Acquired Phototrophy in Aquatic Protists
Vol. 57: 279–310, 2009 AQUATIC MICROBIAL ECOLOGY Printed December 2009 doi: 10.3354/ame01340 Aquat Microb Ecol Published online November 24, 2009 Contribution to AME Special 3 ‘Rassoulzadegan at Villefranche-sur-Mer: 3 decades of aquatic microbial ecology’ OPENPEN ACCESSCCESS Acquired phototrophy in aquatic protists Diane K. Stoecker1,*, Matthew D. Johnson2, Colomban de Vargas3, Fabrice Not3 1University of Maryland Center for Environmental Science, Horn Point Laboratory, PO Box 775, 2020 Horns Point Rd, Cambridge, Maryland 21613, USA 2Woods Hole Oceanographic Institution, Watson Building 109, MS#52, Woods Hole, Massachusetts 02543, USA 3Evolution du Plancton et PaleOceans (EPPO) Lab., UMR 7144 - CNRS & Univ. Paris VI, Station Biologique de Roscoff - SBR, Place George Teissier, BP 74, 29682 Roscoff, Cedex, France ABSTRACT: Acquisition of phototrophy is widely distributed in the eukaryotic tree of life and can involve algal endosymbiosis or plastid retention from green or red origins. Species with acquired phototrophy are important components of diversity in aquatic ecosystems, but there are major differ- ences in host and algal taxa involved and in niches of protists with acquired phototrophy in marine and freshwater ecosystems. Organisms that carry out acquired phototrophy are usually mixotrophs, but the degree to which they depend on phototrophy is variable. Evidence suggests that ‘excess car- bon’ provided by acquired phototrophy has been important in supporting major evolutionary innova- tions that are crucial to the current ecological roles of these protists in aquatic ecosystems. Acquired phototrophy occurs primarily among radiolaria, foraminifera, ciliates and dinoflagellates, but is most ecologically important among the first three. Acquired phototrophy in foraminifera and radiolaria is crucial to their contributions to carbonate, silicate, strontium, and carbon flux in subtropical and trop- ical oceans. -
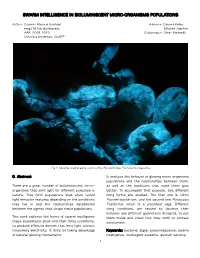
Swarm Intelligence in Bioluminiscent Micro-Organisms Populations
SWARM INTELLIGENCE IN BIOLUMINISCENT MICRO-ORGANISMS POPULATIONS Author: Eduardo Mayoral González Advisors: Edward Keller [email protected] Mitchell Joachim AAR, 2009_2010 Collaborator: Oliver Medvedik Columbia University, GSAPP Fig.1: Glowing dead prawns colonized by Pseudomonas Fluorescence bacteria 0. Abstract It analyzes the behavior of glowing micro-organisms populations and the relationships between them, There are a great number of bioluminescent micro- as well as the conditions that make them glow organisms that emit light for different purposes in better. To accomplish that purpose, two different nature. They form populations that show varied living forms are studied. The first one is Vibrio light emission features depending on the conditions Fischeri bacterium, and the second one Pyrocystis they live in and the relationships established Fusiformis, which is a unicellular alga. Different between the agents that shape these populations. living conditions are tested to observe their behavior and different geometries designed, to put This work explores the forms of swarm intelligence them inside and check how they work to extract these populations show and their living conditions, conclusions. to produce effective devices that emit light without consuming electricity. It does so taking advantage Keywords: bacteria, algae, bioluminescence, swarm of natural glowing mechanisms. intelligence, multiagent systems, quorum sensing. 1 1. Glowing micro-organisms. Design possibilities. BioMario (Fig.3) is a design proposal developed by a team at the University of Osaka for the IGEM Bacteria can be genetically modified to glow using 2009 competition. It uses genetically modified GFP (Green Fluorescent Protein) (Fig.2). Then they glowing bacteria to display an image of the emit light after being exposed to it.