Antimicrobial Peptides: Classification, Design, Application and Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prokaryotes (Domains Bacteria & Archaea)
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection
Published online: 2020-04-12 THIEME 302 A Champion of Host Defense: A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection Martin J. Page, BSc (Hons)1 Etheresia Pretorius, PhD1 1 Department of Physiological Sciences, Stellenbosch University, Address for correspondence Etheresia Pretorius, PhD, Department of Stellenbosch, South Africa Physiological Sciences, Faculty of Science, Stellenbosch University, Private Bag X1 Matieland, Stellenbosch, 7602, South Africa Semin Thromb Hemost 2020;46:302–319. (e-mail: [email protected]). Abstract Thrombocytopenia is commonly associated with sepsis and infections, which in turn are characterized by a profound immune reaction to the invading pathogen. Platelets are one of the cellular entities that exert considerable immune, antibacterial, and antiviral actions, and are therefore active participants in the host response. Platelets are sensitive to surrounding inflammatory stimuli and contribute to the immune response by multiple mechanisms, including endowing the endothelium with a proinflammatory phenotype, enhancing and amplifying leukocyte recruitment and inflammation, promoting the effector functions of immune cells, and ensuring an optimal adaptive immune response. During infection, pathogens and their products influence the platelet response and can even be toxic. However, platelets are able to sense and engage bacteria and viruses to assist in their removal and destruction. Platelets greatly contribute to host defense by multiple mechanisms, including forming immune complexes and aggregates, shedding their granular content, and internalizing pathogens and subsequently being marked for removal. These processes, and the nature of platelet function in general, cause the platelet to be irreversibly consumed in Keywords the execution of its duty. An exaggerated systemic inflammatory response to infection ► platelets can drive platelet dysfunction, where platelets are inappropriately activated and face ► virus immunological destruction. -

A Novel Secretion and Online-Cleavage Strategy for Production of Cecropin a in Escherichia Coli
www.nature.com/scientificreports OPEN A novel secretion and online- cleavage strategy for production of cecropin A in Escherichia coli Received: 14 March 2017 Meng Wang 1, Minhua Huang1, Junjie Zhang1, Yi Ma1, Shan Li1 & Jufang Wang1,2 Accepted: 23 June 2017 Antimicrobial peptides, promising antibiotic candidates, are attracting increasing research attention. Published: xx xx xxxx Current methods for production of antimicrobial peptides are chemical synthesis, intracellular fusion expression, or direct separation and purifcation from natural sources. However, all these methods are costly, operation-complicated and low efciency. Here, we report a new strategy for extracellular secretion and online-cleavage of antimicrobial peptides on the surface of Escherichia coli, which is cost-efective, simple and does not require complex procedures like cell disruption and protein purifcation. Analysis by transmission electron microscopy and semi-denaturing detergent agarose gel electrophoresis indicated that fusion proteins contain cecropin A peptides can successfully be secreted and form extracellular amyloid aggregates at the surface of Escherichia coli on the basis of E. coli curli secretion system and amyloid characteristics of sup35NM. These amyloid aggregates can be easily collected by simple centrifugation and high-purity cecropin A peptide with the same antimicrobial activity as commercial peptide by chemical synthesis was released by efcient self-cleavage of Mxe GyrA intein. Here, we established a novel expression strategy for the production of antimicrobial peptides, which dramatically reduces the cost and simplifes purifcation procedures and gives new insights into producing antimicrobial and other commercially-viable peptides. Because of their potent, fast, long-lasting activity against a broad range of microorganisms and lack of bacterial resistance, antimicrobial peptides (AMPs) have received increasing attention1. -

Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme
Killing of gram-negative bacteria by lactoferrin and lysozyme. R T Ellison 3rd, T J Giehl J Clin Invest. 1991;88(4):1080-1091. https://doi.org/10.1172/JCI115407. Research Article Although lactoferrin has antimicrobial activity, its mechanism of action is not full defined. Recently we have shown that the protein alters the Gram-negative outer membrane. As this membrane protects Gram-negative cells from lysozyme, we have studied whether lactoferrin's membrane effect could enhance the antibacterial activity of lysozyme. We have found that while each protein alone is bacteriostatic, together they can be bactericidal for strains of V. cholerae, S. typhimurium, and E. coli. The bactericidal effect is dose dependent, blocked by iron saturation of lactoferrin, and inhibited by high calcium levels, although lactoferrin does not chelate calcium. Using differing media, the effect of lactoferrin and lysozyme can be partially or completely inhibited; the degree of inhibition correlating with media osmolarity. Transmission electron microscopy shows that E. coli cells exposed to lactoferrin and lysozyme at 40 mOsm become enlarged and hypodense, suggesting killing through osmotic damage. Dialysis chamber studies indicate that bacterial killing requires direct contact with lactoferrin, and work with purified LPS suggests that this relates to direct LPS-binding by the protein. As lactoferrin and lysozyme are present together in high levels in mucosal secretions and neutrophil granules, it is probable that their interaction contributes to host defense. Find the latest version: https://jci.me/115407/pdf Killing of Gram-negative Bacteria by Lactofernn and Lysozyme Richard T. Ellison III*" and Theodore J. Giehl *Medical and tResearch Services, Department of Veterans Affairs Medical Center, and Division ofInfectious Diseases, Department ofMedicine, University ofColorado School ofMedicine, Denver, Colorado 80220 Abstract (4). -

Effect of Dermcidin, an Antimicrobial Peptide, on Body Fat Mobilization in Normal Mice
111 Effect of dermcidin, an antimicrobial peptide, on body fat mobilization in normal mice Kyung-Ah Kim1,*, Sun-O Ka1,*, Woo Sung Moon2, Ho-Keun Yi3, Young-Hoon Lee4, Kang-Beom Kwon5, Jin-Woo Park1 and Byung-Hyun Park1 Departments of 1Biochemistry and 2Pathology, Medical School and Institute for Medical Sciences, 3Biochemistry and 4Oral Anatomy, Dental School and Institute of Oral Biosciences, Chonbuk National University, Jeonju, Jeonbuk 561-756, South Korea 5Department of Physiology, School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, South Korea (Correspondence should be addressed to B-H Park; Email: [email protected]) *(K-A Kim and S-O Ka contributed equally to this work) Abstract Dermcidin (DCD), an antimicrobial peptide that is secreted tissues obtained from Ad-b-gal-injected mice. The gene by sweat glands, is reportedly a human homolog of mouse expression profiles revealed an upregulation of hormone- proteolysis-inducing factor. This study was conducted to sensitive lipase and adipose fatty acid-binding protein, both of investigate the effect of DCD on body fat mobilization. The which are involved in adipocyte lipolysis, in Ad-DCD- expression level of DCD in the livers of Ad-DCD-injected injected mice, and this lipolytic effect of DCD paralleled the mice was higher than in those of Ad-b-galactosidase (Ad-b- increase of circulating tumor necrosis factor-a (TNF-a)level gal)-injected mice 7 days after injection. In addition, injection that was observed. The perilipin levels in adipose tissue were with the Ad-DCD virus led to decreased body weight and decreased in Ad-DCD-injected mice when compared with epididymal fat mass when compared with controls. -

Novel Therapeutic Interventions Towards Improved Management of Septic Arthritis Jian Wang1* and Liucai Wang2
Wang and Wang BMC Musculoskeletal Disorders (2021) 22:530 https://doi.org/10.1186/s12891-021-04383-6 REVIEW Open Access Novel therapeutic interventions towards improved management of septic arthritis Jian Wang1* and Liucai Wang2 Abstract Septic arthritis (SA) represents a medical emergency that needs immediate diagnosis and urgent treatment. Despite aggressive treatment and rapid diagnosis of the causative agent, the mortality and lifelong disability, associated with septic arthritis remain high as close to 11%. Moreover, with the rise in drug resistance, the rates of failure of conventional antibiotic therapy have also increased. Among the etiological agents frequently isolated from cases of septic arthritis, Staphylococcus aureus emerges as a dominating pathogen, and to worsen, the rise in methicillin- resistant S. aureus (MRSA) isolates in bone and joint infections is worrisome. MRSA associated cases of septic arthritis exhibit higher mortality, longer hospital stay, and higher treatment failure with poorer clinical outcomes as compared to cases caused by the sensitive strain i.e methicillin-sensitive S. aureus (MSSA). In addition to this, equal or even greater damage is imposed by the exacerbated immune response mounted by the patient’s body in a futile attempt to eradicate the bacteria. The antibiotic therapy may not be sufficient enough to control the progression of damage to the joint involved thus, adding to higher mortality and disability rates despite the prompt and timely start of treatment. This situation implies that efforts and focus towards studying/ understanding new strategies for improved management of sepsis arthritis is prudent and worth exploring. The review article aims to give a complete insight into the new therapeutic approaches studied by workers lately in this field. -
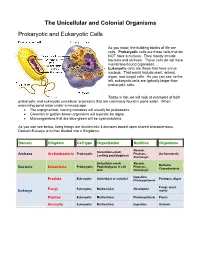
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

Design, Development, and Characterization of Novel Antimicrobial Peptides for Pharmaceutical Applications Yazan H
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 8-2013 Design, Development, and Characterization of Novel Antimicrobial Peptides for Pharmaceutical Applications Yazan H. Akkam University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/etd Part of the Biochemistry Commons, Medicinal and Pharmaceutical Chemistry Commons, and the Molecular Biology Commons Recommended Citation Akkam, Yazan H., "Design, Development, and Characterization of Novel Antimicrobial Peptides for Pharmaceutical Applications" (2013). Theses and Dissertations. 908. http://scholarworks.uark.edu/etd/908 This Dissertation is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Design, Development, and Characterization of Novel Antimicrobial Peptides for Pharmaceutical Applications Design, Development, and Characterization of Novel Antimicrobial Peptides for Pharmaceutical Applications A Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Cell and Molecular Biology by Yazan H. Akkam Jordan University of Science and Technology Bachelor of Science in Pharmacy, 2001 Al-Balqa Applied University Master of Science in Biochemistry and Chemistry of Pharmaceuticals, 2005 August 2013 University of Arkansas This dissertation is approved for recommendation to the Graduate Council. Dr. David S. McNabb Dissertation Director Professor Roger E. Koeppe II Professor Gisela F. Erf Committee Member Committee Member Professor Ralph L. Henry Dr. Suresh K. Thallapuranam Committee Member Committee Member ABSTRACT Candida species are the fourth leading cause of nosocomial infection. The increased incidence of drug-resistant Candida species has emphasized the need for new antifungal drugs. -

Resistance to Host Antimicrobial Peptides Is Necessaryfor
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 11939-11943, December 1992 Genetics Resistance to host antimicrobial peptides is necessary for Salmonella virulence (transposon mutagenesis/defensin/magainin/cecropin/pathogenesis) EDUARDO A. GROISMAN*t, CARLOS PARRA-LOPEZ*, MARGARITA SALCEDO*, CRAIG J. Lippst, AND FRED HEFFRON*§ *Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110; tDepartment of Molecular Biology, Research Institute of Scripps Clinic, La Jolla, CA 92037; and §Department of Microbiology and Immunology, Oregon Health Sciences University, Portland, OR 97201 Communicated by David M. Kipnis, September 14, 1992 ABSTRACT The production of antibacterial peptides is a distinct strategies to evade killing by the phagocyte oxygen- host defense strategy used by various species, including mam- dependent and -independent mechanisms (3). mals, amphibians, and insects. Successful pathogens, such as To cause disease, Salmonella must withstand the battery of the facultative intracellular bacterium Salmonella typhimu- short peptides with antibiotic activity present in phagocytic num, have evolved resistance mechanisms to this ubiquitous cells and other tissues. One such group of peptides is the type of host defense. To identify the genes required for resis- defensins, which are abundant in the azurophilic granules of tance to host peptides, we isolated a library of 20,000 MudJ neutrophils and macrophages from rabbits, rats, guinea pigs, transposon insertion mutants of a virulent peptide-resistant S. and humans and the crypt cells of mouse intestine (4). The typhimurium strain and screened it for hypersensitivity to the importance of these peptides for host defense is underscored antimicrobial peptide protamine. Eighteen mutants had by the fact that patients with specialized granule deficiency- heightened susceptibility to protamine and 12 of them were who lack defensins-have recurrent infections (5). -

Analysis of the Solution Structure of the Human Antibiotic Peptide Dermcidin and Its Interaction with Phospholipid Vesicles
BMB reports Analysis of the solution structure of the human antibiotic peptide dermcidin and its interaction with phospholipid vesicles Hyun Ho Jung1, Sung-Tae Yang2, Ji-Yeong Sim1, Seungkyu Lee1, Ju Yeon Lee1, Ha Hyung Kim3, Song Yub Shin4 & Jae Il Kim1,* 1Department of Life Science, Gwangju Institute of Science and Technology, Gwangju, 2Section on Membrane Biology, Laboratory of Cellular and Molecular Biophysics, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, 3College of Pharmacy, Chung-Ang University, Seoul, 4Department of Bio-Materials, Graduate School and Department of Cellular & Molecular Medicine, School of Medicine, Chosun University, Gwangju 501-759, Korea Dermcidin is a human antibiotic peptide that is secreted by the of the modes of action of various antimicrobial peptides have sweat glands and has no homology to other known antimicro- revealed that their cationic nature contributes to their initial bial peptides. As an initial step toward understanding dermci- binding to negatively charged bacterial membranes through din’s mode of action at bacterial membranes, we used homo- electrostatic interaction, while their amphipathic structures en- nuclear and heteronuclear NMR to determine the conforma- hance peptide-lipid interactions at the water-bilayer interface, tion of the peptide in 50% trifluoroethanol solution. We found ultimately leading to cell death via pore formation or mem- that dermcidin adopts a flexible amphipathic α-helical struc- brane disintergration (9-14). ture with a helix-hinge-helix motif, which is a common molec- Dermcidin (DCD) is an antimicrobial peptide found in hu- ular fold among antimicrobial peptides. Spin-down assays of man sweat. -

Peptides to Tackle Leishmaniasis: Current Status and Future Directions
International Journal of Molecular Sciences Review Peptides to Tackle Leishmaniasis: Current Status and Future Directions Alberto A. Robles-Loaiza 1, Edgar A. Pinos-Tamayo 1, Bruno Mendes 2,Cátia Teixeira 3 , Cláudia Alves 3 , Paula Gomes 3 and José R. Almeida 1,* 1 Biomolecules Discovery Group, Universidad Regional Amazónica Ikiam, Tena 150150, Ecuador; [email protected] (A.A.R.-L.); [email protected] (E.A.P.-T.) 2 Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas (UNICAMP), Campinas 13083-862, Brazil; [email protected] 3 LAQV-REQUIMTE, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade do Porto, 4169-007 Porto, Portugal; [email protected] (C.T.); [email protected] (C.A.); [email protected] (P.G.) * Correspondence: [email protected] Abstract: Peptide-based drugs are an attractive class of therapeutic agents, recently recognized by the pharmaceutical industry. These molecules are currently being used in the development of innovative therapies for diverse health conditions, including tropical diseases such as leishmaniasis. Despite its socioeconomic influence on public health, leishmaniasis remains long-neglected and categorized as a poverty-related disease, with limited treatment options. Peptides with antileishmanial effects encountered to date are a structurally heterogeneous group, which can be found in different natural sources—amphibians, reptiles, insects, bacteria, marine organisms, mammals, plants, and others—or inspired by natural toxins or proteins. This review details the biochemical and structural characteris- Citation: Robles-Loaiza, A.A.; tics of over one hundred peptides and their potential use as molecular frameworks for the design of Pinos-Tamayo, E.A.; Mendes, B.; antileishmanial drug leads. -

Anticancer Potential of Bioactive Peptides from Animal Sources (Review)
ONCOLOGY REPORTS 38: 637-651, 2017 Anticancer potential of bioactive peptides from animal sources (Review) LiNghoNg WaNg1*, CHAO DONG2*, XIAN LI1, WeNyaN haN1 and XIULAN SU1 1Clinical Medicine Research Center of the affiliated hospital, inner Mongolia Medical University; 2College of Basic Medicine of Inner Mongolia Medical University, Huimin, Hohhot, Inner Mongolia 010050, P.R. China Received October 10, 2016; Accepted April 10, 2017 DOI: 10.3892/or.2017.5778 Abstract. Cancer is the most common cause of human death 3. Mechanisms of action of bioactive peptides underlying worldwide. Conventional anticancer therapies, including their anticancer effects chemotherapy and radiation, are associated with severe side 4. Summary and perspective effects and toxicities as well as low specificity. Peptides are rapidly being developed as potential anticancer agents that specifically target cancer cells and are less toxic to normal 1. Introduction tissues, thus making them a better alternative for the preven- tion and management of cancer. Recent research has focused Although the rates of death due to cancer have been continu- on anticancer peptides from natural animal sources, such ously declining for the past 2 decades in developed nations, as terrestrial mammals, marine animals, amphibians, and cancer remains a major public health threat in many parts of animal venoms. However, the mode of action by which bioac- the world (1). The incidence of cancer in the developing world tive peptides inhibit the proliferation of cancer cells remains is currently increasing. Specifically, 55% of new cases arise unclear. In this review, we present the animal sources from in developing nations, a figure that could reach 60% by 2020 which bioactive peptides with anticancer activity are derived and 70% by 2050.