Supplemental Data Set 1 FPKM Expression for Immune Checkpoint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening and Identification of Key Biomarkers in Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Screening and identification of key biomarkers in clear cell renal cell carcinoma based on bioinformatics analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Clear cell renal cell carcinoma (ccRCC) is one of the most common types of malignancy of the urinary system. The pathogenesis and effective diagnosis of ccRCC have become popular topics for research in the previous decade. In the current study, an integrated bioinformatics analysis was performed to identify core genes associated in ccRCC. An expression dataset (GSE105261) was downloaded from the Gene Expression Omnibus database, and included 26 ccRCC and 9 normal kideny samples. Assessment of the microarray dataset led to the recognition of differentially expressed genes (DEGs), which was subsequently used for pathway and gene ontology (GO) enrichment analysis. -

The TNF and TNF Receptor Review Superfamilies: Integrating Mammalian Biology
Cell, Vol. 104, 487±501, February 23, 2001, Copyright 2001 by Cell Press The TNF and TNF Receptor Review Superfamilies: Integrating Mammalian Biology Richard M. Locksley,*²³k Nigel Killeen,²k The receptors and ligands in this superfamily have and Michael J. Lenardo§k unique structural attributes that couple them directly to *Department of Medicine signaling pathways for cell proliferation, survival, and ² Department of Microbiology and Immunology differentiation. Thus, they have assumed prominent ³ Howard Hughes Medical Institute roles in the generation of tissues and transient microen- University of California, San Francisco vironments. Most TNF/TNFR SFPs are expressed in the San Francisco, California 94143 immune system, where their rapid and potent signaling § Laboratory of Immunology capabilities are crucial in coordinating the proliferation National Institute of Allergy and Infectious Diseases and protective functions of pathogen-reactive cells. National Institutes of Health Here, we review the organization of the TNF/TNFR SF Bethesda, Maryland 20892 and how these proteins have been adapted for pro- cesses as seemingly disparate as host defense and or- ganogenesis. In interpreting this large and highly active Introduction area of research, we have focused on common themes that unite the actions of these genes in different tissues. Three decades ago, lymphotoxin (LT) and tumor necro- We also discuss the evolutionary success of this super- sis factor (TNF) were identified as products of lympho- familyÐsuccess that we infer from its expansion across cytes and macrophages that caused the lysis of certain the mammalian genome and from its many indispens- types of cells, especially tumor cells (Granger et al., able roles in mammalian biology. -

Human TNFRSF18 ELISA Kit (ARG81453)
Product datasheet [email protected] ARG81453 Package: 96 wells Human TNFRSF18 ELISA Kit Store at: 4°C Component Cat. No. Component Name Package Temp ARG81453-001 Antibody-coated 8 X 12 strips 4°C. Unused strips microplate should be sealed tightly in the air-tight pouch. ARG81453-002 Standard 2 X 10 ng/vial 4°C ARG81453-003 Standard/Sample 30 ml (Ready to use) 4°C diluent ARG81453-004 Antibody conjugate 1 vial (100 µl) 4°C concentrate (100X) ARG81453-005 Antibody diluent 12 ml (Ready to use) 4°C buffer ARG81453-006 HRP-Streptavidin 1 vial (100 µl) 4°C concentrate (100X) ARG81453-007 HRP-Streptavidin 12 ml (Ready to use) 4°C diluent buffer ARG81453-008 25X Wash buffer 20 ml 4°C ARG81453-009 TMB substrate 10 ml (Ready to use) 4°C (Protect from light) ARG81453-010 STOP solution 10 ml (Ready to use) 4°C ARG81453-011 Plate sealer 4 strips Room temperature Summary Product Description ARG81453 Human TNFRSF18 ELISA Kit is an Enzyme Immunoassay kit for the quantification of Human TNFRSF18 in serum, plasma (heparin, EDTA) and cell culture supernatants. Tested Reactivity Hu Tested Application ELISA Specificity There is no detectable cross-reactivity with other relevant proteins. Target Name TNFRSF18 Conjugation HRP Conjugation Note Substrate: TMB and read at 450 nm. Sensitivity 31.25 pg/ml Sample Type Serum, plasma (heparin, EDTA) and cell culture supernatants. Standard Range 62.5 - 4000 pg/ml Sample Volume 100 µl www.arigobio.com 1/3 Precision Intra-Assay CV: 6.3% Inter-Assay CV: 7.0% Alternate Names Tumor necrosis factor receptor superfamily member 18; AITR; CD357; CD antigen CD357; Activation- inducible TNFR family receptor; GITR-D; GITR; Glucocorticoid-induced TNFR-related protein Application Instructions Assay Time ~ 5 hours Properties Form 96 well Storage instruction Store the kit at 2-8°C. -

An Ontogenetic Switch Drives the Positive and Negative Selection of B Cells
An ontogenetic switch drives the positive and negative selection of B cells Xijin Xua, Mukta Deobagkar-Lelea, Katherine R. Bulla, Tanya L. Crockforda, Adam J. Meadb, Adam P. Cribbsc, David Simsc, Consuelo Anzilottia, and Richard J. Cornalla,1 aMedical Research Council Human Immunology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; bMedical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; and cMedical Research Council, Weatherall Institute of Molecular Medicine, Centre for Computational Biology, Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom Edited by Michael Reth, University of Freiburg, Freiburg, Germany, and approved January 6, 2020 (received for review September 3, 2019) + Developing B cells can be positively or negatively selected by self- BM HSCs increased CD5 B-1a B cell development (15), while antigens, but the mechanisms that determine these outcomes are expression of let-7b in FL pro-B cells blocked the development of incompletely understood. Here, we show that a B cell intrinsic B-1 B cells (17). These findings support the notion of hard-wired switch between positive and negative selection during ontogeny differences during ontogeny, but possibly downstream of the HSC is determined by a change from Lin28b to let-7 gene expression. commitment stage. Ectopic expression of a Lin28b transgene in murine B cells restored Several lines of evidence also suggest that B-1 B cells can un- the positive selection of autoreactive B-1 B cells by self-antigen in dergo positive selection, which is linked to their B cell receptor adult bone marrow. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

B Cell Checkpoints in Autoimmune Rheumatic Diseases
REVIEWS B cell checkpoints in autoimmune rheumatic diseases Samuel J. S. Rubin1,2,3, Michelle S. Bloom1,2,3 and William H. Robinson1,2,3* Abstract | B cells have important functions in the pathogenesis of autoimmune diseases, including autoimmune rheumatic diseases. In addition to producing autoantibodies, B cells contribute to autoimmunity by serving as professional antigen- presenting cells (APCs), producing cytokines, and through additional mechanisms. B cell activation and effector functions are regulated by immune checkpoints, including both activating and inhibitory checkpoint receptors that contribute to the regulation of B cell tolerance, activation, antigen presentation, T cell help, class switching, antibody production and cytokine production. The various activating checkpoint receptors include B cell activating receptors that engage with cognate receptors on T cells or other cells, as well as Toll-like receptors that can provide dual stimulation to B cells via co- engagement with the B cell receptor. Furthermore, various inhibitory checkpoint receptors, including B cell inhibitory receptors, have important functions in regulating B cell development, activation and effector functions. Therapeutically targeting B cell checkpoints represents a promising strategy for the treatment of a variety of autoimmune rheumatic diseases. Antibody- dependent B cells are multifunctional lymphocytes that contribute that serve as precursors to and thereby give rise to acti- cell- mediated cytotoxicity to the pathogenesis of autoimmune diseases -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

A20 Regulates the Therapeutic Effect of Anti-PD-1 Immunotherapy In
Open access Original research J Immunother Cancer: first published as 10.1136/jitc-2020-001866 on 9 December 2020. Downloaded from A20 regulates the therapeutic effect of anti- PD-1 immunotherapy in melanoma Weinan Guo,1 Jinyuan Ma,1 Sen Guo,1 Huina Wang,1 Sijia Wang,1,2 Qiong Shi,1 Lin Liu,1 Tao Zhao,1 Fengfan Yang,3 Shuyang Chen,4 Jianru Chen,1 Jianhong Zhao,1 Chen Yu,1 Xiuli Yi,1 Yuqi Yang,1 Jingjing Ma,1 Qingrong Ni,1 Guannan Zhu,1 1 1 Tianwen Gao, Chunying Li To cite: Guo W, Ma J, Guo S, ABSTRACT (PD-1)/programmed death ligand 1 (PD-L1) et al. A20 regulates the Background The therapeutic effect of immune have been demonstrated as a pair of major therapeutic effect of anti- PD-1 checkpoint blockers, especially the neutralizing immunotherapy in melanoma. immune checkpoint molecules and valuable antibodies of programmed cell death (PD-1) and its ligand 1 Journal for ImmunoTherapy therapeutic targets for melanoma treatment. programmed death ligand 1 (PD-L1), has been well verified of Cancer 2020;8:e001866. For instance, the binding of membrane doi:10.1136/jitc-2020-001866 in melanoma. Nevertheless, the dissatisfactory response rate and the occurrence of resistance significantly hinder PD- L1 on tumor cells to PD-1 on T cells the treatment effect. Inflammation- related molecules like evokes an immunosuppressive signal that ► Additional material is results in the dysfunction and even the apop- published online only. To view A20 are greatly implicated in cancer immune response, please visit the journal online but the role of tumorous A20 in antitumor immunity and tosis of cytotoxic T cells, thereby impairing 2 (http:// dx. -

Human Neonatal B Cell Immunity Differs from the Adult Version by Conserved Ig Repertoires and Rapid, but Transient Response Dynamics
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.11.245985; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Human neonatal B cell immunity differs from the adult version by conserved Ig repertoires and rapid, but transient response dynamics Bettina Budeus,1,# Artur Kibler,1,# Martina Brauser,1,# Ekaterina Homp,1,# Kevin Bronischewski,1 J. Alexander Ross,1 Andre Görgens,2,3 Marc A. Weniger,1 Josefine Dunst,4,5 Taras Kreslavsky,4,5 Symone Vitoriano da Conceição Castro,2,6 Florian Murke,2 Christopher C. Oakes,7,8 Peter Rusch,9 Dimitrios Andrikos,9 Peter Kern,9 Angela Köninger,9 Monika Lindemann,2 Patricia Johansson,10 Wiebke Hansen,11 Anna-Carin Lundell,12 Anna Rudin,12 Jan Dürig,10 Bernd Giebel,2 Daniel Hoffmann,13 Ralf Küppers,1 Marc Seifert1* 1Institute of Cell Biology (Cancer Research), Medical Faculty, University of Duisburg-Essen, 45147 Essen, Germany. 2Institute for Transfusion Medicine, Medical Faculty, University of Duisburg-Essen, Essen 45147, Germany. 3Department of Laboratory Medicine, Karolinska Institute, Stockholm 17177, Sweden. 4Division of Immunology and Allergy, Department of Medicine Solna, Karolinska Institute, Karolinska University Hospital, Stockholm 17177, Sweden. 5Center for Molecular Medicine, Karolinska Institute, Stockholm 17177, Sweden 6CAPES Foundation, Ministry of Education of Brazil, Brasília 70610-908, Brazil. 7Department of Internal Medicine, Division of Hematology, The Ohio State University, Columbus, OH 43210, USA. 8Department of Biomedical Informatics, The Ohio State University, Columbus, OH 43210, USA. 9Department of Gynecology, Medical Faculty, University of Duisburg- Essen, Essen 45147, Germany. -

Combination Immune Therapy
Combination Immune Therapy Translational Medicine Plenary SWOG April 17, 2017 San Francisco T‐cell Activation, Proliferation, and Function is Controlled by Multiple Agonist and Antagonist Signals 1. Co‐stimulation via CD28 ligation 2. CTLA‐4 ligation on activated T 3. T cell function in tissue is subject transduces T cell activating signals cells down‐regulates T cell to feedback inhibition responses T cell Functional Block T cell Activation T cell Activation T cell Proliferative Block T cell T cell APC T cell CTLA‐4 T cell CTLA‐4 TCR TCR PD‐1 PD‐1 PD‐1 IFN‐gamma TCR CD28 Cytokines TCR CD4 MHC MHC PD‐‐L1 MHC B7 MHC CD28 B7 PD‐‐L1 Tumor or Tumor or APC APC Immune cell B7 immune cell APC NK Presence of PD-L1 or TILs1 PPD-L1 /TIL+ PD-L1/TIL + + PD-L1 /TIL+ + PD-L1 /TIL PD-L1 /TIL D-L1 /TIL+ Schalper and Rimm, Yale University NSCLC PD-L1/TIL 45% 17% 26% 12% Type 1 Type 2 Type 3 Type 4 45% 41% 13% 1% Melanoma45% 17% 26% 12% Taube et al Type 1 Type 2 Type 3 Type 4 Tumor‐specific T cells are contained in the PD‐1+ TIL population and are functional after in vitro culture Spectrum of PD‐1/PD‐L1 Antagonist Activity Active • Melanoma Minimal to no activity • Renal cancer (clear cell and non‐clear cell) • Prostate cancer • NSCLC – adenocarcinoma and squamous cell • MMR+ (MSS) colon cancer • Small cell lung cancer • Myeloma • Head and neck cancer • Pancreatic cancer • Gastric and gastroesophageal junction • MMR‐repair deficient tumors (colon, cholangiocarcinoma) • Bladder • Triple negative breast cancer • Ovarian Major PD‐1/PD‐L1 antagonists • Hepatocellular -
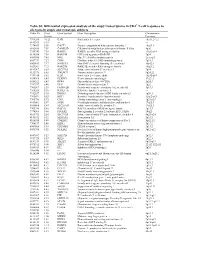
Table S1| Differential Expression Analysis of the Atopy Transcriptome
Table S1| Differential expression analysis of the atopy transcriptome in CD4+ T-cell responses to allergens in atopic and nonatopic subjects Probe ID S.test Gene Symbol Gene Description Chromosome Statistic Location 7994280 10.32 IL4R Interleukin 4 receptor 16p11.2-12.1 8143383 8.95 --- --- --- 7974689 8.50 DACT1 Dapper, antagonist of beta-catenin, homolog 1 14q23.1 8102415 7.59 CAMK2D Calcium/calmodulin-dependent protein kinase II delta 4q26 7950743 7.58 RAB30 RAB30, member RAS oncogene family 11q12-q14 8136580 7.54 RAB19B GTP-binding protein RAB19B 7q34 8043504 7.45 MAL Mal, T-cell differentiation protein 2cen-q13 8087739 7.27 CISH Cytokine inducible SH2-containing protein 3p21.3 8000413 7.17 NSMCE1 Non-SMC element 1 homolog (S. cerevisiae) 16p12.1 8021301 7.15 RAB27B RAB27B, member RAS oncogene family 18q21.2 8143367 6.83 SLC37A3 Solute carrier family 37 member 3 7q34 8152976 6.65 TMEM71 Transmembrane protein 71 8q24.22 7931914 6.56 IL2R Interleukin 2 receptor, alpha 10p15-p14 8014768 6.43 PLXDC1 Plexin domain containing 1 17q21.1 8056222 6.43 DPP4 Dipeptidyl-peptidase 4 (CD26) 2q24.3 7917697 6.40 GFI1 Growth factor independent 1 1p22 7903507 6.39 FAM102B Family with sequence similarity 102, member B 1p13.3 7968236 5.96 RASL11A RAS-like, family 11, member A --- 7912537 5.95 DHRS3 Dehydrogenase/reductase (SDR family) member 3 1p36.1 7963491 5.83 KRT1 Keratin 1 (epidermolytic hyperkeratosis) 12q12-q13 7903786 5.72 CSF1 Colony stimulating factor 1 (macrophage) 1p21-p13 8019061 5.67 SGSH N-sulfoglucosamine sulfohydrolase (sulfamidase) 17q25.3 -

TNFRSF18 (Human) ELISA Kit
TNFRSF18 (Human) ELISA Kit Catalog Number KA1720 96 assays Version: 01 Intended for research use only www.abnova.com I. INTRODUCTION GITR (glucocorticoid induced tumor necrosis factor receptor family related gene) is a type-1 transmembrane protein of 228 amino acids belonging to the TNF and NGF receptor family of proteins. GITR is expressed in normal T-lymphocytes from thymus, spleen. Constitutive expression of a transfected GITR gene induces resistance to apoptosis induced by anti CD3 monoclonal antibodies. The human homolog of GITR is expressed in lymph node and peripheral blood leukocytes. Its expression is up-regulated in human peripheral mononuclear cells mainly after stimulation with antibodies against CD3 and CD28. TNFRSF18 (Human) ELISA Kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of human GITR in cell lysate and tissue lysate. This assay employs an antibody specific for human GITR coated on a 96-well plate. Standards and samples are pipetted into the wells and GITR present in a sample is bound to the wells by the immobilized antibody. The wells are washed and biotinylated anti-human GITR antibody is added. After washing away unbound biotinylated antibody, HRP-conjugated streptavidin is pipetted to the wells. The wells are again washed, a TMB substrate solution is added to the wells and color develops in proportion to the amount of GITR bound. The Stop Solution changes the color from blue to yellow, and the intensity of the color is measured at 450 nm. II. REAGENTS 1. GITR Microplate (Item A): 96 wells (12 strips x 8 wells) coated with anti-human GITR.