Extraocular Muscle Forces in Normal Human Subjects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
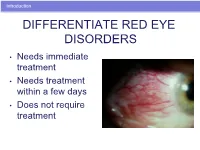
Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

Evaluation of the Eyebrow Position After External Müller's Muscle
Journal of Plastic, Reconstructive & Aesthetic Surgery (2019) 72, 662–668 Evaluation of the eyebrow position after external Müller’s muscle tucking: A new technique for ptosis repair a , ∗ b c Kenichi Kokubo , Nobutada Katori , Kengo Hayashi , d e f a Jun Sugawara , Seiko Kou , Akiko Fujii , Shoko Haga , f Jiro Maegawa a Department of Plastic Surgery, Fujisawa Shounandai Hospital. 2345 Takakura, Fujisawa-shi, Kanagawa 251-0802, Japan b Department of Ocular Plastic & Orbital Surgery, Seirei Hamamatsu General Hospital. 2-12-12 Sumiyoshi, Naka-ku, Hamamatsu-shi, Shizuoka 430-8558, Japan c Yokohama Sakuragicho Eye Clinic. 1-200 Hinodecho, Naka-ku Yokohama-shi, Kanagawa 231-0006, Japan d JUN CLINIC, 1402-5 Kitaishidocho, Nagano-shi, Nagano 380-0826, Japan e KO CLINIC for Antiaging. 4-54 Onoecho, Naka-ku Yokohama-shi, Kanagawa 231-0015, Japan f Department of Plastic and Reconstructive Surgery, Yokohama City University Hospital. 3-9 Fukuura, Kanazawa-ku, Yokohama-shi, Kanagawa 236-0004, Japan Received 27 August 2018; accepted 6 January 2019 KEYWORDS Summary Eyebrow descent commonly occurs after ptosis repair or blepharoplasty surgery. Müller’s muscle; The procedures used to correct acquired blepharoptosis are primarily classified into four Eyebrow position; groups. These procedures target the levator aponeurosis, Müller’s muscle, both the aponeu- Blepharoptosis; rosis and Müller’s muscle, or the frontalis muscle. In this study, we used a new technique called MRD; external Müller’s muscle tucking (EMMT) on 51 patients (94 eyelids), which targets the Müller’s Ptosis repair muscle for involutional blepharoptosis. The patients were assessed by comparative analysis us- ing pre- and post-operative digital photographs. -

Treatment of Congenital Ptosis
13 Review Article Page 1 of 13 Treatment of congenital ptosis Vladimir Kratky1,2^ 1Department of Ophthalmology, Queen’s University, Kingston, Canada; 21st Medical Faculty, Charles University, Prague, Czech Republic Correspondence to: Vladimir Kratky, BSc, MD, FRCSC, DABO. Associate Professor of Ophthalmology, Director of Ophthalmic Plastic and Orbital Surgery, Oculoplastics Fellowship Director, Queen’s University, Kingston, Canada; 1st Medical Faculty, Charles University, Prague, Czech Republic. Email: [email protected]. Abstract: Congenital ptosis is an abnormally low position of the upper eyelid, with respect to the visual axis in the primary gaze. It can be present at birth or manifest itself during the first year of life and can be bilateral or unilateral. Additionally, it may be an isolated finding or part of a constellation of signs of a specific syndrome or systemic associations. Depending on how much it interferes with the visual axis, it may be considered as a functional or a cosmetic condition. In childhood, functional ptosis can lead to deprivation amblyopia and astigmatism and needs to be treated. However, even mild ptosis with normal vision can lead to psychosocial problems and correction is also advised, albeit on a less urgent basis. Although, patching and glasses can be prescribed to treat the amblyopia, the mainstay of management is surgical. There are several types of surgical procedure available depending on the severity and etiology of the droopy eyelid. The first part of this paper will review the different categories of congenital ptosis, including more common associated syndromes. The latter part will briefly cover the different surgical approaches, with emphasis on how to choose the correct condition. -

Conjunctival Flora of Normal Human Eye Which Vary with Age, Sex, Geographical Distribution, Right and Left Eye
Central JSM Ophthalmology Research Article *Corresponding author Purnima Rajkarnikar Sthapit, Department of Ophthalmology, Dhulikhel Hospital, Kathmandu Conjunctival Flora of Normal University Hospital, Dhulikhel, Kavre, Nepal, Tel: 009779813254962; Fax: 0097711490707; Email: Human Eye Submitted: 23 February 2014 Purnima Rajkarnikar Sthapit1* and Nhuchhe Ratna Tuladhar2 Accepted: 03 March 2014 1Department of Ophthalmology, Kathmandu University School of Medical Sciences, Nepal Published: 07 March 2014 2Department of Microbiology, Kathmandu University School of Medical Sciences, Nepal ISSN: 2333-6447 Copyright Abstract © 2014 Sthapit et al. Background: The normal flora of the eye plays an important role in maintaining OPEN ACCESS ocular homeostasis by various mechanisms. They comprise of mainly bacteria which do not cause infection in normal conditions but can be a main source of infection after Keywords ocular surgery, trauma or in immune compromised. The ranges of these microorganisms • Coagulase positive Staphylococcus vary with age, sex and geographical distribution. Therefore it is very important for the • Normal flora ophthalmologist to know the ocular normal flora before giving prophylactic antibiotics • Ocular infection and treating infections. • Ocular trauma Objectives: To describe the conjunctival flora of normal human eye which vary with age, sex, geographical distribution, right and left eye. Methodology: A total of 200 conjunctival swabs from 100 patients with healthy eyes were sent for microbiological evaluation to describe the various microorganisms isolated as normal flora of conjunctiva. Result: The growth of bacteria was seen in 78.5% of patients, the commonest flora isolated was Coagulase negative Staphylocccus in 51%. Greater number of male patients had sterile conjunctiva than females and conjunctiva of old people were found to be increasingly more colonised than young. -

Eyelid Conjunctival Tumors
EYELID &CONJUNCTIVAL TUMORS PHOTOGRAPHIC ATLAS Dr. Olivier Galatoire Dr. Christine Levy-Gabriel Dr. Mathieu Zmuda EYELID & CONJUNCTIVAL TUMORS 4 EYELID & CONJUNCTIVAL TUMORS Dear readers, All rights of translation, adaptation, or reproduction by any means are reserved in all countries. The reproduction or representation, in whole or in part and by any means, of any of the pages published in the present book without the prior written consent of the publisher, is prohibited and illegal and would constitute an infringement. Only reproductions strictly reserved for the private use of the copier and not intended for collective use, and short analyses and quotations justified by the illustrative or scientific nature of the work in which they are incorporated, are authorized (Law of March 11, 1957 art. 40 and 41 and Criminal Code art. 425). EYELID & CONJUNCTIVAL TUMORS EYELID & CONJUNCTIVAL TUMORS 5 6 EYELID & CONJUNCTIVAL TUMORS Foreword Dr. Serge Morax I am honored to introduce this Photographic Atlas of palpebral and conjunctival tumors,which is the culmination of the close collaboration between Drs. Olivier Galatoire and Mathieu Zmuda of the A. de Rothschild Ophthalmological Foundation and Dr. Christine Levy-Gabriel of the Curie Institute. The subject is now of unquestionable importance and evidently of great interest to Ophthalmologists, whether they are orbital- palpebral specialists or not. Indeed, errors or delays in the diagnosis of tumor pathologies are relatively common and the consequences can be serious in the case of malignant tumors, especially carcinomas. Swift diagnosis and anatomopathological confirmation will lead to a treatment, discussed in multidisciplinary team meetings, ranging from surgery to radiotherapy. -

A Rare Presentation of Lower Conjunctival Fornix Eyelashes Cyst Dr Mohammad Aldroos (MD)* *Department of Ophthalmology, Jordanian Royal Medical Services
Int J Biol Med Res.2018 ;9(1):6259-6260 Int J Biol Med Res www.biomedscidirect.com Volume 6, Issue 2, April 2015 Contents lists available at BioMedSciDirect Publications International Journal of Biological & Medical Research Journal homepage: www.biomedscidirect.com BioMedSciDirect International Journal of Publications BIOLOGICAL AND MEDICAL RESEARCH Case report A rare presentation of lower conjunctival fornix eyelashes cyst Dr Mohammad Aldroos (MD)* *Department of Ophthalmology, Jordanian Royal Medical Services A R T I C L E I N F O A B S T R A C T Keywords: Purpose: To describe a rare presentation of a right lower conjunctival fornix eyelashes cyst. Case Report: A 56 year-old lady presented to our clinic complaining of blackish mass in the lower conjunctival fornix of the right eye ( see fig.1), with mild conjunctival hyperemia around the mass. There was no history of discharge and difficulty in extraocular muscles movements also there was no history of trauma. A gradually enlarging mass over the past few years with recurrent conjunctivitis. Slit lamp examination of the mass showed normal appearing eyelashes surrounded by capsule only. The mass was surgically excised with good cosmetic appearance and without any complications c Copyright 2010 BioMedSciDirect Publications IJBMR - ISSN: 0976:6685. All rights reserved. Introduction Fig.2 Eyelashes grow on the edge of the eyelid. A normal eyelid has a single row of eyelashes located along its anterior margin. The posterior portion of the lid contains a row of Meibomian glands orifices, which secrete the oily component of the tear film (1). There are approximately 100 eye lashes on the upper lid and approximately 50-75 on the lower lid, they protect the eyeball. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Orbital Imaging in Thyroid-Related Orbitopathy
Orbital imaging in thyroid-related orbitopathy Christopher Lo, MD, Shoaib Ugradar, MD, and Daniel Rootman, MD, MS SUMMARY A broad understanding of the different imaging modalities used to assess the physiologic changes seen in Graves’ orbitopathy complement clinical examination. Subtle applications of radiographic imaging techniques allow for a better understanding of the overall physiology of the orbit, quantify progression of disease, and differentiate it from orbital diseases with overlapping features. A nuanced approach to interpreting imaging features may allow us to delineate inactive from active thyroid eye disease, and advances within this field may arm clinicians with the ability to better predict and prevent dysthyroid optic neuropathy. ( J AAPOS 2018;22:256.e1-256.e9) rbital imaging plays a central role in the diag- mean inferior rectus width, 4.8 mm; medial rectus width, nosis and management of thyroid-related orbit- 4.2 mm; superior rectus width, 4.6 mm; and lateral rectus O opathy (TRO). Diagnostically, it is used to width, 3.3 mm.8,9 These numbers can be used as a guide; compliment a careful ophthalmic examination, laboratory however, they represent population averages, each with values, and ancillary studies to confirm the presence of significant variation. Overlap in populations exist, and TRO and/or dysthyroid optic neuropathy (DON). It can both diseased and nondiseased muscles can have widths also be helpful in surgical planning and understanding close to these values. In the end, there are no strict rules. the progression of thyroid myopathy. Computed tomogra- In terms of muscle involvement, clinical myopathy is phy (CT), magnetic resonance imaging (MRI), ultrasound, thought to most often involve the inferior rectus muscle, and nuclear medicine all have applications in the field. -

Extraconjunctival Approach to the Extra-Ocular Muscles* by M
Br J Ophthalmol: first published as 10.1136/bjo.38.8.497 on 1 August 1954. Downloaded from Brit. J. Ophthal. (1954) 38, 497. EXTRACONJUNCTIVAL APPROACH TO THE EXTRA-OCULAR MUSCLES* BY M. SARWAR Oxford SINCE contact lenses have been used for monocular conditions of impaired vision in which the eye is divergent, e.g., aphakia and corneal scars, it has been found that although vision could be improved the affected eye remained divergent. Some of these eyes, i.e. those with small degrees of divergence, attained single binocular vision with orthoptic treatment; others with larger angles had to be helped surgically. Orthodox trans-conjunctival surgery on these muscles was not very suitable for the following reasons: (i) The majority of lenses which can be worn with comfort and without corneal haze require large haptic surfaces and need to be ventilated. (ii) The haptic surfaces of these lenses extend beyond the sites of insertion of the muscles, and any scarring of this area naturally upsets the fit and balance of the lenses, rendering them unwearable. (iii) In certain cases, although the lens might still be wearable, the haze-free time is copyright. shortened. (iv) In yet other cases, the ventilating bubble increases in size owing to the disturbance in the fit, thus interfering with clear sight. To overcome these difficulties an attempt was made to reduce the diameter of the lenses, but this was found to upset their balance. The contact lens in these cases has to be fitted before any operative intervention in order to obtain the full benefit of restored visual acuity and orthoptic treatment; the residual http://bjo.bmj.com/ divergence only can then be treated surgically. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Eye External Anatomy of Eye Accessory Structures
4/22/16 Eye Bio 40B Dr. Kandula External Anatomy of Eye Accessory Structures l Eyebrows l Levator Palpebrae Superioris - opens eye l Eyelashes l Ciliary glands – modified sweat glands l Small sebaceous glands l Sty is inflamed ciliary glands or small sebaceous glands 1 4/22/16 Terms: Lacrimal gland and duct Surface of eye Lacrimal puncta Lacrimal sac Nasolacrimal duct Nasal cavity Tears / Lacrimal fluid l a watery physiologic saline, with a plasma-like consistency, l contains the bactericidal enzyme lysozyme; l it moistens the conjunctiva and cornea, l provides nutrients and dissolved O2 to the cornea. Extrinsic Muscles of the Eye: Lateral/medial rectus Important to know Superior/inferior rectus actions and nerve Superior/inferior oblique supply in table 2 4/22/16 Extrinsic Eye Muscles • Eye movements controlled by six extrinsic eye muscles Four recti muscles § Superior rectus – moves eyeball superiorly supplied by Cranial Nerve III § Inferior rectus - moves eyeball inferiorly supplied by Cranial Nerve III § Lateral rectus - moves eyeball laterally supplied by Cranial Nerve VI § Medial rectus - moves eyeball medially supplied by Cranial Nerve III Extrinsic Eye Muscles Two oblique muscles rotate eyeball on its axis § Superior oblique rotates eyeball inferiorly and laterally and is supplied by Cranial Nerve IV § Inferior oblique rotates superiorly and laterally and is supplied by Cranial Nerve III Convergence of the Eyes l Binocular vision in humans has both eyes looking at the same object l As you look at an object close to your face, -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos.