Imperata Cylindrica) Populations in Mississippi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Biosystematic Study of the Genus Imperata (Gramineae: Andropogoneae) Mark Lauren Gabel Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1982 A biosystematic study of the genus Imperata (Gramineae: Andropogoneae) Mark Lauren Gabel Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Gabel, Mark Lauren, "A biosystematic study of the genus Imperata (Gramineae: Andropogoneae) " (1982). Retrospective Theses and Dissertations. 7499. https://lib.dr.iastate.edu/rtd/7499 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting througli an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Revised Survey for Special-Status Vascular Plant Species
REVISED SURVEY FOR SPECIAL-STATUS VASCULAR PLANT SPECIES For the proposed Deer Creek Irrigation District Fish Passage Improvement Project Tehama County, California Prepared for: Tehama Environmental Solutions 910 Main Street, Suite D Red Bluff, California 96080 Prepared by: Dittes & Guardino Consulting P.O. Box 6 Los Molinos, California 96055 (530) 384-1774 [email protected] Deer Creek DCID Dam Fish Passage Project - Botany Report January 22, 2019 Prepared by: Dittes & Guardino Consulting 1 REVISED SURVEY FOR SPECIAL-STATUS VASCULAR PLANT SPECIES Deer Creek DCID Dam Fish Passage Project Tehama County, California T25N, R1W, NW1/4 Sec. 23, NE1/4 Sec. 22 of the Acorn Hollow 7.5’ USGS Topographic Quadrangle & T25N, R1W, E1/2 Sec. 27 of the Richardson Springs NW 7.5’ USGS Topographic Quadrangle TABLE OF CONTENTS I. Executive Summary ................................................................................................................................................. 4 II. Introduction ............................................................................................................................................................ 4 III. Project Description ............................................................................................................................................... 5 IV. Location .................................................................................................................................................................. 5 V. Methods .................................................................................................................................................................. -
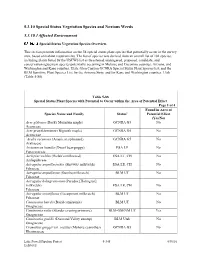
Special Status Vegetation Species and Noxious Weeds
5.3.10 Special Status Vegetation Species and Noxious Weeds 5.3.10.1 Affected Environment Special Status Vegetation Species Overview. This section presents information on the 58 special status plant species that potentially occur in the survey area, based on habitat requirements. The list of species was derived from an overall list of 101 species, including plants listed by the USFWS list as threatened, endangered, proposed, candidate, and conservation agreement species potentially occurring in Mohave and Coconino counties, Arizona, and Washington and Kane counties, Utah; Glen Canyon GCNRA Special Status Plant Species list; and the BLM Sensitive Plant Species List for the Arizona Strip, and for Kane and Washington counties, Utah (Table 5-88). Table 5-88 Special Status Plant Species with Potential to Occur within the Area of Potential Effect Page 1 of 4 Found in Area of Species Name and Family Status1 Potential Effect (Yes/No) Acer glabrum (Rocky Mountain maple) GCNRA G5 No Aceraceae Acer grandidentatum (Bigtooth maple) GCNRA G5 No Aceraceae `Aralia racemosa (American spikenard) GCNRA G5 No Araliaceae Arctomecon humilis (Dwarf bear-poppy) ESA LE No Papaveraceae Asclepias welshii (Welsh’s milkweed) ESA LT, CH No Asclepidaceae Astragalus ampullarioides (Shivwits milkvetch) ESA LE, CH No Fabaceae Astragalus ampullarius (Gumbo milkvetch) BLM UT No Fabaceae Astragalus holmgreniorum (Paradox [Holmgren] milkvetch) ESA LE, CH No Fabaceae Astragalus striatiflorus (Escarpment milkvetch) BLM UT No Fabaceae Camissonia bairdii (Baird camissonia) BLM -

Sentinel 09-28-18
The San Bernardino County News of Note from Around the Largest County in the Lower 48 States Friday, SeptemberSentinel 28, 2018 A Fortunado Publication in conjunction with Countywide News Service 10808 Foothill Blvd. Suite 160-446 Rancho Cucamonga, CA 91730 (951) 567-1936 Over Intense Opposition, Board OKs Putting Warehouse Among Houses Petition Effort By Mark Gutglueck Board of Supervisors on included an amendment the negative consequenc- ing network of activists Over the objections of Tuesday voted 4-to-1 to to the county’s general es of the warehouse’s de- and environmentalists To Halt Nestlé’s two members of the Cal- allow residential proper- plan, the granting of a velopment and therefore on the other. But there SB Mountain ifornia legislature and ty in the unincorporated conditional use permit, justified granting the remains yet the possibil- dozens of residents who county area of Bloom- the certification of a final proponent permission to ity that a lawsuit will be H20 Diversion were speaking that day, ington adjoining a school environmental impact proceed with the project. filed on behalf of the un- By Amanda Frye and more than two dozen and other residences to report which enumerated County officials are incorporated county ar- Mark Gutglueck residents who inveighed be rezoned for light in- negative consequences hoping the vote will ea’s residents to prevent In recent years, there against the project in dustrial use so it can host for nearby residents and bring to an end the four- J.M. Realty Group, Inc. has been significant con- person five weeks pre- a 344,000-square foot students attending an ad- year controversy that has from proceeding with troversy over the Nestlé viously and hundreds of warehouse. -

Classification and Biogeography of Panicoideae (Poaceae) in the New World Fernando O
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Scholarship@Claremont Aliso: A Journal of Systematic and Evolutionary Botany Volume 23 | Issue 1 Article 39 2007 Classification and Biogeography of Panicoideae (Poaceae) in the New World Fernando O. Zuloaga Instituto de Botánica Darwinion, San Isidro, Argentina Osvaldo Morrone Instituto de Botánica Darwinion, San Isidro, Argentina Gerrit Davidse Missouri Botanical Garden, St. Louis Susan J. Pennington National Museum of Natural History, Smithsonian Institution, Washington, D.C. Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Zuloaga, Fernando O.; Morrone, Osvaldo; Davidse, Gerrit; and Pennington, Susan J. (2007) "Classification and Biogeography of Panicoideae (Poaceae) in the New World," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 23: Iss. 1, Article 39. Available at: http://scholarship.claremont.edu/aliso/vol23/iss1/39 Aliso 23, pp. 503–529 ᭧ 2007, Rancho Santa Ana Botanic Garden CLASSIFICATION AND BIOGEOGRAPHY OF PANICOIDEAE (POACEAE) IN THE NEW WORLD FERNANDO O. ZULOAGA,1,5 OSVALDO MORRONE,1,2 GERRIT DAVIDSE,3 AND SUSAN J. PENNINGTON4 1Instituto de Bota´nica Darwinion, Casilla de Correo 22, Labarde´n 200, San Isidro, B1642HYD, Argentina; 2([email protected]); 3Missouri Botanical Garden, PO Box 299, St. Louis, Missouri 63166, USA ([email protected]); 4Department of Botany, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20013-7012, USA ([email protected]) 5Corresponding author ([email protected]) ABSTRACT Panicoideae (Poaceae) in the New World comprise 107 genera (86 native) and 1357 species (1248 native). -

Clark County Multiple Species Habitat Conservation Plan Amendment Covered Species Analysis Report
Clark County Multiple Species Habitat Conservation Plan Amendment Covered Species Analysis Report Prepared For: Clark County Department of Air Quality Desert Conservation Program 4701 West Russell Blvd., Suite 200 Las Vegas, NV 89118 WRA Contact: Ken Sanchez (415) 578-3184 [email protected] Patricia Valcarcel (415) 524-7542 [email protected] Date: June 11, 2018 WRA Project: 26346 2169-G East Francisco Blvd., San Rafael, CA 94702 (415) 454-8868 tel [email protected] www.wra-ca.com Draft Covered Species Analysis Report – June 2018 This page intentionally left blank. Draft Covered Species Analysis Report – June 2018 TABLE OF CONTENTS 1.0 INTRODUCTION.................................................................................................................. 1 2.0 SPECIES REVISION PROCESS ......................................................................................... 2 2.1 Species Considered for Coverage ............................................................................ 2 2.2 Criteria for Covered Species ..................................................................................... 3 3.0 ANALYSIS ........................................................................................................................... 4 3.1 Species Range ......................................................................................................... 4 3.2 Species Status.......................................................................................................... 4 3.3 Impacts from Covered Activities .............................................................................. -

Studi Etnobotani Pemanfaatan Tumbuhan Upacara Adat Suku Dayak Tunjung Di Kabupaten Kutai Barat Provinsi Kalimantan Timur
PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI STUDI ETNOBOTANI PEMANFAATAN TUMBUHAN UPACARA ADAT SUKU DAYAK TUNJUNG DI KABUPATEN KUTAI BARAT PROVINSI KALIMANTAN TIMUR SKRIPSI Diajukan untuk Memenuhi Salah Satu Syarat Memperoleh Gelar Sarjana Pendidikan Program Studi Pendidikan Biologi Oleh: Yeri Lona 091434028 PROGRAM STUDI PENDIDIKAN BIOLOGI JURUSAN PENDIDIKAN MATEMATIKA DAN ILMU PENGETAHUAN ALAM FAKULTAS KEGURUAN DAN ILMU PENDIDIKAN UNIVERSITAS SANATA DHARMA YOGYAKARTA 2014 PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI ii PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI iii PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI “Kata-kata dalam tulisan adalah kuat” Karya Ilmiah ini saya persembahkan kepada Ayah dan Ibu tercinta yang telah mendedikasikan seluruh hidup mereka demi tercapainya cita-cita yang saya inginkan. iv PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI v PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI vi PLAGIATPLAGIAT MERUPAKAN MERUPAKAN TINDAKAN TINDAKAN TIDAK TIDAK TERPUJI TERPUJI ABSTRAK Budaya tradisional Suku Dayak Tunjung di Kutai Barat yang kaya akan berbagai kearifan lokal dan berperan aktif dalam pelestarian lingkungan belum banyak diungkap dan didata kedalam bentuk tulisan, khususnya pemanfaatan tumbuh-tumbuhan untuk proses upacara adat. Suku Dayak Tunjung terdiri dari beberapa Sub-suku, diantaranya adalah Suku Dayak -
Appendix A: Biological Resources Analysis
City of Hemet—Downtown Hemet Specific Plan Project Initial Study/Mitigated Negative Declaration Appendix A: Biological Resources Analysis FirstCarbon Solutions Y:\Publications\Client (PN-JN)\2266\22660027\ISMND\22660027 Hemet Downtown SP ISMND.docx THIS PAGE INTENTIONALLY LEFT BLANK Memo Date: November 8, 2016 Ms. Crystal Robinson, Procurement Administrator Hemet City Hall—Planning Division To: 445 E. Florida Avenue Hemet, CA 92543-4209 From: Ashley Laor, Associate Biologist Subject: Downtown Hemet Specific Plan Project—Biologgical Resources Analysis FirstCarbon Solutions (FCS) conducted a biological resources aassessment to document the existing biological conditions and analyze potential impacts to biological resources within the Specific Plan area. This letter report evaluates the existing biological resources found on-site. Project Location and Description The Specific Plan area is located in the city of Hemet, Riverside County, California. The project plan area is bordered by Oakland Avenue on the north, Gilbert Street on the west, Acacia Avenue on the south, and Santa Fe Street on the east (Exhibit 1). The Specific Plan’’s total area is 360 acres. The City is presently developing a Specific Plan; the proposed Specific Plan is a comprehensive plan that features a land use plan, circulation plan, urban design framework, utility infrastructure plan, development standards, design guidelines, and sustainability plan for futuure development within the plan boundaries. The Specific Plan would remain consistent with the community’s vision for the City and individual projects would be required to comply with all federal, state, and local regulations. Regulatory Framework This section provides an overview of the laws and regulations that influence biological resources. -
Appendix B: General Biological Resources Assessment
Chino Francis Appendices APPENDIX B: GENERAL BIOLOGICAL RESOURCES ASSESSMENT City of Chino Draft IS/MND October 2019 Memorandum Date: April 23, 2019 To: Konnie Dobreva, EPD Solutions, Inc. From: Juan J. Hernandez Subject: Updated Biological Assessment for Francis Avenue Residential Development Project located in the City of Chino, San Bernardino County, California Pursuant to your request, Hernandez Environmental Services (HES) conducted a biological assessment to document existing biological conditions associated with development of the Francis Avenue Residential Development Project site, consisting of approximately 13.35 acres located at 4570 Francis Avenue in the City of Chino, San Bernardino County, California (Figures 1 and 2). The proposed Francis Avenue Residential Development Project would result in the construction of a residential development and associated infrastructure on the entire 13.35-acre site. The project site is located within the City of Chino. The site encompasses an approximate 13.35-acre area generally located north of the intersection of Francis Avenue and Yorba Avenue. The project site consists of roofed animal pens, concrete pads, and a storage building. The site is relatively flat with an elevation of approximately 850 feet above mean sea level (amsl). A single soil type, Tujunga loamy sand, occurs on the site. The project site is surrounded by residential land uses to the east, south, and west. A General Biological Resources Assessment was prepared for the project by Psomas in July of 2016 (Appendix B). According to the previous study, the project site consists of developed and disturbed areas. The developed areas consist of roofed animal pens, concrete pads, and a storage building The disturbed areas consist of those areas with no concrete or pavement, exhibiting bare soil or sparse vegetation subject to vegetation management pursuant to Chapter 15.32 (specifically, Section 504.1.2 Vegetation) of the City of Chino Municipal Code. -

Nevada Springs Conservation Plan Acknowledgements
Nevada SpriNgS Conservation Plan ackNowledgemeNtS We would like to thank the Nevada Division of State Lands Conservation and Resource Protection Grant Program, known as ‘Question 1 Program’ and the U.S. Environmental Protection Agency for funding the development of this Springs Conservation Plan. Without the support of Nevada voters who passed Question 1 in 2002 to ‘protect, preserve, and obtain the benefits of the property and natural resources of this state’, this effort to support conservation of Nevada’s aquatic biodiversity would not have been possible. The Springs Conservation Plan working group dedicated time and travel to multiple workshops to ensure the content of this plan represented a multi-agency effort. Special thanks to Don Sada, Jennifer Newmark, Victor Cobos, and Bob Conrad for their extra time and commitment that facilitated the integration of the data collection and planning components of this project. Finally, we would like to acknowledge the individuals who reviewed this Plan and provided valuable comments. Recommended Citation: Abele, S.L. (ed.) 2011. Nevada Springs Conservation Plan. Springs Conservation Plan Working Group. The Nature Conservancy, Reno, NV. Cover photo: Charnock Ranch, Nevada. © Janel Johnson about this rePort introduction ................................3 How do we determine if a Spring is Healthy? ..................9 what are the concerns for the Future? ......................... 15 taking action to conserve Nevada’s Springs ................... 23 Significant Spring landscapes ..............................27 Next Steps for Springs conservation in Nevada.........36 references ............................... 39 appendix 1 ............................... 41 Ash Springs, Pahranagat Valley, NV. © Christiana Manville appendix 2 ................................47 he purpose of this Plan is to summarize the current condition, identify future threats, and highlight necessary actions to con- serve some of nevada’s most significant aquatic environments. -

Systematics of California Grasses (Poaceae)
TWO Systematics of California Grasses (Poaceae) PAUL M. PETERSON AND ROBERT J. SORENG The grass family (Poaceae or Gramineae) is the fourth largest relationships among organisms) of the major tribes of flowering plant family in the world and contains about California grasses. 11,000 species in 800 genera worldwide. Twenty-three gen- era contain 100 or more species or about half of all grass Morphology species, and almost half of the 800 genera are monotypic or diatypic, i.e., with only one or two species (Watson and The most important feature of grasses (Poaceae) is a one- Dallwitz 1992, 1999). seeded indéhiscent fruit (seed coat is fused with the ovary Over the last 150 years the grass flora of California has wall), known as a caryopsis or grain (see Figure 2.1; Peterson been the subject of considerable attention by botanists. 2003). The grain endosperm is rich in starch, although it can Bolander (1866) prepared the first comprehensive list, recog- contain protein and significant quantities of lipids. The nizing 112 grasses from California, of which 31 were intro- embryo is located on the basal portion of the caryopsis and ductions. Thurber (1880) mentions 175 grasses in California, contains high levels of protein, fats, and vitamins. The stems and Beetle (1947) enumerates 400 known species. It is inter- are referred to as culms, and the roots are fibrous and princi- esting to note that Crampton (1974) recognized 478 grasses pally adventitious or arising from lower portions of the in California, and of these, 175 were introduced and 156 were culms. Silica-bodies are a conspicuous component of the reported as annuals (we report 152 annuals here). -

Gall Midge Orseolia Javanica (Diptera: Cecidomyiidae), a Candidate Biological Control Agent of Cogongrass
Gall Midge Orseolia javanica (Diptera: Cecidomyiidae), a Candidate Biological Control Agent of Cogongrass James P. Cuda1 and William A. Overholt2 1Entomology & Nematology Department, Gainesville, FL 32611 [email protected] 2Retired, BioControl Research & Containment Laboratory, Ft. Pierce, FL 34945 [email protected] Acknowledgements • Millie Burrell • Patricia Kline • Purnama Hidayat ______ FWC, Bureau of Invasive Plant Management Outline • Background on Cogongrass • Potential for Biological Control • Research on Orseolia javanica • Cogongrass IPM Model • Questions and Comments Outline • Background on Cogongrass • Potential for Biological Control • Research on Orseolia javanica • Cogongrass IPM Model • Questions and Comments Cogongrass • Imperata cylindrica (L.) Raeuschel (Poaceae) • Federal Listed Noxious Weed • Established in southeastern US as forage grass • Invasive in Alabama, Mississippi, & Florida Cogongrass Impacts • Displaces native/desirable vegetation • Evidence of allelopathy • Increases frequency and severity of fires • Perennial; 4 clonal types • Rhizotomous (60% of biomass in rhizome) • C4 photosynthesis http://bugwood.blogspot.com/2013/05/cogongrass-in- georgia-spring-2013-update.html Worldwide Distribution World distribution of Imperata cylindrica based on the Global Biodiversity Information Facility (www.gbif.org). US Distribution (www.eddmaps.org) Genetic Diversity of US Cogongrass 15 Florida peninsula 10 Gulf Coast 5 Philippines 0 Aiken, SC -10 -5 0 5 10 15 20 25 Second axis Japanese blood grass -5 Imperata brasiliensis -10 -15 First axis Burrell, M., A. E. Pepper, G. Hodnett, J. A. Goolsby, W. A. Overholt, A. E. Racelis, R. Diaz and P. E. Klein. 2015. Exploring origins, invasion history and genetic diversity of Imperata cylindrica (L.) P. Beauv. (Cogongrass) in the United States using genotyping by sequencing. Molecular Ecology. DOI: 10.1111/mec.13167.