Chemoembolization for Hepatic Tumors Effective Date: 7/15/2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spontaneous Hemothorax Caused by a Ruptured Intercostal Artery Aneurysm in Von Recklinghausen’S Neurofibromatosis
W.C. Chang, H.H. Hsu, H. Chang, et al BRIEF COMMUNICATION SPONTANEOUS HEMOTHORAX CAUSED BY A RUPTURED INTERCOSTAL ARTERY ANEURYSM IN VON RECKLINGHAUSEN’S NEUROFIBROMATOSIS Wei-Chou Chang,1 Hsian-He Hsu,1 Hung Chang,2 and Cheng-Yu Chen1 Abstract: Aneurysms arising from an intercostal artery are very rare vascular malformations in von Recklinghausen’s neurofibromatosis, which often have a silent clinical presentation and are difficult to diagnose before rupture. We report a case of von Recklinghausen’s neurofibromatosis with massive hemothroax caused by spontaneous rupture of an intercostal artery aneurysm in a 29-year-old man. The diagnosis was eventually confirmed by percutaneous angiography and treated with endovascular embolization. During a 10-month follow-up period, the patient had a satisfactory recovery. This case illustrates that angiography and possible endovascular embolization should be the first strategy in managing hemothorax in patients with von Recklinghausen’s disease. Key words: Aneurysm, ruptured; Embolization, therapeutic; Hemothorax; Neurofibromatosis 1; Thoracic arteries J Formos Med Assoc 2005;104:286-9 Von Recklinghausen’s neurofibromatosis, also named neurofibromatosis type 1 (NF-1), is a well-recognized Case Report entity that is characterized by numerous neuro- fibromas, spots of abnormal cutaneous pigmentation, A 29-year-old man presented at the emergency room and a variety of other dysplastic abnormalities of with sudden onset shortness of breath and severe the skin, nervous system, bones, endocrine organs and retrosternal pain radiating to his back. One week prior blood vessels.1,2 Vascular abnormalities in patients to admission, dull discomfort upon breathing was with NF-1 have been described for large, medium- noted, which was not affected by position or physical sized and small muscular arteries in the intracranial, activity. -
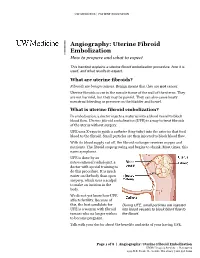
Uterine Fibroid Embolization Procedure, How It Is Used, and What Results to Expect
UW MEDICINE | PATIENT EDUCATION | | Angiography: Uterine Fibroid | Embolization | How to prepare and what to expect This handout explains a uterine fibroid embolization procedure, how it is used, and what results to expect. What are uterine fibroids? Fibroids are benign tumors. Benign means that they are not cancer. Uterine fibroids occur in the muscle tissue of the wall of the uterus. They are not harmful, but they may be painful. They can also cause heavy menstrual bleeding or pressure on the bladder and bowel. What is uterine fibroid embolization? In embolization, a doctor injects a material into a blood vessel to block blood flow. Uterine fibroid embolization (UFE) is a way to treat fibroids DRAFTof the uterus without surgery. UFE uses X-rays to guide a catheter (tiny tube) into the arteries that feed blood to the fibroid. Small particles are then injected to block blood flow. With its blood supply cut off, the fibroid no longer receives oxygen and nutrients. The fibroid stops growing and begins to shrink. Most times, this eases symptoms. UFE is done by an interventional radiologist, a doctor with special training to do this procedure. It is much easier on the body than open surgery, which uses a scalpel to make an incision in the body. We do not yet know how UFE affects fertility. Because of this, the best candidate for During UFE, small particles are injected UFE is a woman with fibroid into blood vessels to block blood flow to tumors who no longer wishes the fibroid. to become pregnant. Talk with your doctor about the benefits and risks of your having UFE. -

Embolization of Brain Aneurysms and Arteriovenous
Embolization of Brain Aneurysms and Arteriovenous Malformations/Fistulas Embolization of brain aneurysms and arteriovenous malformations (AVM) uses imaging guidance to place small, soft metal coils into an aneurysm to block the flow of blood and prevent the aneurysm from rupturing. It also is used to fill AVMs – abnormal connections between arteries and veins – with liquid embolic agents (similar to fast-sealing glue). AVMs may prevent oxygenated blood from completely circulating throughout the brain and can cause a variety of problems, including headache, weakness, and other neurological symptoms. Embolization treats cerebral aneurysms and AVMs previously thought inoperable and is much less invasive than open surgery. Your doctor will instruct you on how to prepare, including any changes to your medication schedule. Tell your doctor if there's a possibility you are pregnant and discuss any recent illnesses, medical conditions, allergies and medications you're taking, including herbal supplements and aspirin. You may be advised to stop taking aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) or blood thinners several days prior to your procedure. You also may be told not to eat or drink anything after midnight before your procedure. Plan to stay at the hospital overnight. Leave jewelry at home and wear loose, comfortable clothing. You will be asked to wear a gown. What is Embolization of Brain Aneurysms and Fistulas? Embolization of brain aneurysms and arteriovenous malformations (AVM)/fistulas is a minimally invasive treatment for aneurysms and other blood vessel malformations that occur in the brain. These problems are typically identified in adults; however, aneurysms and AVMs can also occur in children. -

Pulmonary Embolism A-Nd Thrombosis R
Postgrad Med J: first published as 10.1136/pgmj.38.435.13 on 1 January 1962. Downloaded from POSTGRAD MED. J. (I962), 38, 13 PULMONARY EMBOLISM A-ND THROMBOSIS R. MARSHALL, M.D., M.R.C.P. Nuffield Department of Surgery, Radcliffe Infirmary, Oxford SUDDEN death following a call for a bedpan is a chest pain and ECG changes when the balloon classical mode of presentation of pulmonary em- was inflated in a smaller artery (Brofman, Charms, bolism, but it is probable that cases of this severity Kohn, Elder, Newman and Rizika, I957). On the form only a small proportion of the total. Pul- other hand, it is a common practice to impact the monary embolism may be caused by blood clot, tip of a cardiac catheter in branches of the pul- fat, cells or foreign bodies, but most commonly by monary artery of about 2 mm. diameter with no blood clot. ill effects. In spite of the undoubted ability of emboli to produce reflex effects under certain cir- cumstances, there is a growing body of evidence The Physiological Effects that the effects of pulmonary embolism by inert The physiological effects ofpulmonary embolism particles are produced by the magnitude of the depend upon the size and number of the emboli mechanical blockage of the pulmonary vessels and and they may produce these effects either by are not dependent upon the size of the emboli or mechanical blockage of the circulation when they reflex vasoconstriction (Daley, I957; McEvoy, are of sufficient size or number, or by the initiation Harder and Dale, 1958; Williams, 1956). -

Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism
ASE GUIDELINES AND STANDARDS Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism Muhamed Saric, MD, PhD, FASE, Chair, Alicia C. Armour, MA, BS, RDCS, FASE, M. Samir Arnaout, MD, Farooq A. Chaudhry, MD, FASE, Richard A. Grimm, DO, FASE, Itzhak Kronzon, MD, FASE, Bruce F. Landeck, II, MD, FASE, Kameswari Maganti, MD, FASE, Hector I. Michelena, MD, FASE, and Kirsten Tolstrup, MD, FASE, New York, New York; Durham, North Carolina; Beirut, Lebanon; Cleveland, Ohio; Aurora, Colorado; Chicago, Illinois; Rochester, Minnesota; and Albuquerque, New Mexico Embolism from the heart or the thoracic aorta often leads to clinically significant morbidity and mortality due to transient ischemic attack, stroke or occlusion of peripheral arteries. Transthoracic and transesophageal echo- cardiography are the key diagnostic modalities for evaluation, diagnosis, and management of stroke, systemic and pulmonary embolism. This document provides comprehensive American Society of Echocardiography guidelines on the use of echocardiography for evaluation of cardiac sources of embolism. It describes general mechanisms of stroke and systemic embolism; the specific role of cardiac and aortic sour- ces in stroke, and systemic and pulmonary embolism; the role of echocardiography in evaluation, diagnosis, and management of cardiac and aortic sources of emboli including the incremental value of contrast and 3D echocardiography; and a brief description of alternative imaging techniques and their role in the evaluation of cardiac sources of emboli. Specific guidelines are provided for each category of embolic sources including the left atrium and left atrial appendage, left ventricle, heart valves, cardiac tumors, and thoracic aorta. In addition, there are recommen- dation regarding pulmonary embolism, and embolism related to cardiovascular surgery and percutaneous procedures. -

Guidelines for Peripheral and Visceral Vascular Embolization Training
Guidelines for Peripheral and Visceral Vascular Embolization Training Joint Writing Groups of the Standards of Practice Committees for the Society of Interventional Radiology (SIR), Cardiovascular and Interventional Radiological Society of Europe (CIRSE), and Canadian Interventional Radiology Association (CIRA) Jafar Golzarian, MD, Marc R. Sapoval, MD, PhD, Sanjoy Kundu, MD, David W. Hunter, MD, Elias N. Brountzos, MD, Jean-Francois H. Geschwind, MD, Timothy P. Murphy, MD, James B. Spies, MD, Michael J. Wallace, MD, Thierry de Baere, MD, and John F. Cardella, MD J Vasc Interv Radiol 2010; 21:436–441 EMBOLOTHERAPY or embolization 1. Vascular malformations: occlusion 3. Trauma: for control of dramatic hem- has rapidly developed in recent years of congenital or acquired aneu- orrhage, for example, related to and now represents an integral part of rysms (cerebral, visceral, extremi- splenic laceration or pelvic fractures. interventional radiology practice. Em- ties), pseudoaneurysms, vascular 4. Uterine artery embolization: devascu- bolotherapy is defined as the percuta- malformations, or other vascular larization of benign uterine leiomyo- neous endovascular application of one abnormalities that have potential mas and adenomyosis for symptom or more of a variety of agents or ma- to cause adverse health effects (1–18). alleviation or to reduce operative terials to accomplish vascular occlu- 2. Nontraumatic hemorrhage: treat- blood loss (3,4,14,54–57). sion. ment of acute or recurrent hem- 5. Oncologic embolization: to relieve Embolotherapy has evolved since its orrhage (eg, hemoptysis, gastro- symptoms, prevent or treat hem- clinical introduction to include a wide intestinal bleeding, postpartum orrhage, reduce operative blood loss, or improve survival and variety of applications that can be and iatrogenic hemorrhage, and quality of life (3,4,14,54–57). -

Endovascular Interventional Radiology of the Urogenital Tract
medicina Review Endovascular Interventional Radiology of the Urogenital Tract Fabio Pozzi Mucelli 1,*, Roberta A. Pozzi Mucelli 1, Cristina Marrocchio 2, Saverio Tollot 2 and Maria A. Cova 2 1 Department of Radiology, ASUGI, Ospedale di Cattinara, 34149 Trieste, Italy; [email protected] 2 Department of Radiology, University of Trieste, ASUGI, Ospedale di Cattinara, 34149 Trieste, Italy; [email protected] (C.M.); [email protected] (S.T.); [email protected] (M.A.C.) * Correspondence: [email protected] Abstract: Interventional radiology of the male urogenital system includes percutaneous and en- dovascular procedures, and these last consist mostly of transcatheter arterial embolizations. At the kidney level, arterial embolizations are performed mainly for palliative treatment of parenchymal tumors, for renal traumas and, less frequently, for arteriovenous fistulas and renal aneurysms and pseudoaneurysms. These latter may often require emergency intervention as they can cause renal or peri-renal hematomas or significant hematuria. Transcatheter arterial embolization is also an effective therapy for intractable severe bladder hematuria secondary to a number of neoplastic and inflammatory conditions in the pelvis, including unresectable bladder cancer and radiation-induced or cyclophosphamide-induced hemorrhagic cystitis. Endovascular interventional procedures for the penis are indicated for the treatment of post-traumatic priapism. In this article, we review the main endovascular radiological interventions of the male urogenital system, describing the technical aspects, results, and complications of each procedure at the various anatomical districts. Citation: Pozzi Mucelli, F.; Pozzi Keywords: embolization; angiography; urogenital system; kidney; bladder; priapism Mucelli, R.A.; Marrocchio, C.; Tollot, S.; Cova, M.A. -

Coil Embolization for Intracranial Aneurysms
Ontario Health Technology Assessment Series 2006; Vol. 6, No. 1 Coil Embolization for Intracranial Aneurysms An Evidence-Based Analysis January 2006 Medical Advisory Secretariat Ministry of Health and Long-Term Care Suggested Citation This report should be cited as follows: Medical Advisory Secretariat. Coil embolization for intracranial aneurysms: an evidence-based analysis. Ontario Health Technology Assessment Series 2006; 6(1) Permission Requests All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to [email protected]. How to Obtain Issues in the Ontario Health Technology Assessment Series All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas. Print copies can be obtained by contacting [email protected]. Conflict of Interest Statement All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare. Peer Review All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html. Contact Information The Medical Advisory Secretariat Ministry of Health and Long-Term Care 20 Dundas Street West, 10th floor Toronto, Ontario CANADA M5G 2N6 Email: [email protected] Telephone: 416-314-1092 ISSN 1915-7398 (Online) ISBN 1-4249-0335-1 (PDF) 2 Coil Embolization - Ontario Health Technology Assessment Series 2006; Vol. -

If You Have Liver Cancer
cancer.org | 1.800.227.2345 EASY READING If You Have Liver Cancer Jump to a topic ● What is liver cancer? ● Different kinds of liver cancer ● How does the doctor know I have liver cancer? ● Tests that might be done ● How serious is my cancer? ● What kind of treatment will I need? What is liver cancer? Liver cancer is a cancer that starts in the liver. It happens when cells in the liver start to change and grow out of control, crowding out normal cells. This makes it hard for the body to work the way it should. The liver makes bile to help the body use food. It also cleans the blood and helps the blood clot when you are cut. Liver cancer cells can spread to other parts of the body. When cancer does this, it is called metastasis. But even if a liver cancer spreads to your bones, it is still called and treated like a liver cancer, not bone cancer. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 The liver Ask your doctor to use this picture to show you where the cancer is. Different kinds of liver cancer Primary liver cancer If a cancer starts in the liver, it is called primary liver cancer. There are many types of liver cancer. Some are very rare. Your doctor can tell you more about the type you have. The most common type of liver cancer is called hepatocellular carcinoma. Also known as HCC. It starts in the cells that make up the liver. 2 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Some rare types of liver cancer are: ● Angiosarcoma ● Hemangiosarcoma These cancers start in the cells lining the blood vessels of the liver. -

Transcatheter Arterial Embolization in Lower Gastrointestinal Bleeding: Ischemia Remains a Concern Even with a Superselective Approach
Journal of Gastrointestinal Surgery (2018) 22:1394–1403 https://doi.org/10.1007/s11605-018-3728-7 ORIGINAL ARTICLE Transcatheter Arterial Embolization in Lower Gastrointestinal Bleeding: Ischemia Remains a Concern Even with a Superselective Approach Taina Nykänen1 & Erno Peltola2 & Leena Kylänpää1 & Marianne Udd1 Received: 15 October 2017 /Accepted: 22 February 2018 /Published online: 16 March 2018 # 2018 The Society for Surgery of the Alimentary Tract Abstract Purpose To evaluate the safety, efficacy, and feasibility of transcatheter arterial embolization (TAE) in the treatment of lower gastrointestinal bleeding (LGIB). Methods Study group comprised all patients receiving angiography for LGIB in the Helsinki University Hospital during the period of 2004–2016. Hospital medical records provided the study data. Rebleeding, complication, and mortality rates (≤ 30 days) were the primary outcomes. Secondary outcomes included need for blood transfusions, durations of intensive care unit and hospital admissions, incidence of delayed rebleeding, and long-term complications, as well as overall survival. Results During the study period, angiography for LGIB was necessary on 123 patients. Out of 123, 55 (45%) underwent embolization attempts. TAE was technically successful in 53 (96%). Rebleeding occurred in 14 (26%). The complication rate was 36%, minor complications occurring in 10 (19%) and major in nine (17%). Major complications resulted in bowel resection in seven (13%). Post embolization ischemia was the most common single complication seen in nine (17%). The mortality rate was 6%. Survival estimates of 1 and 5 years were 79 and 49%. Discussion LGIB is a severe physiological insult occurring in patients who are often elderly and moribund. Although major post embolization complications occur, transcatheter arterial embolization should be the first-line approach over surgery in profuse LGIB in patients with hemodynamic instability, when colonoscopy fails or is unavailable, or when computerized tomography angiography detects small intestinal bleeding. -

Trans-Arterial Embolization of Renal Cell Carcinoma Prior to Percutaneous Ablation: Technical Aspects, Institutional Experience, and Brief Review of the Literature
Surgical Technique Curr Urol 2018;12:43–49 Received: November 25, 2017 DOI: 10.1159/000447230 Accepted: November 29, 2017 Published online: June 30, 2018 Trans-Arterial Embolization of Renal Cell Carcinoma prior to Percutaneous Ablation: Technical Aspects, Institutional Experience, and Brief Review of the Literature Andrew J. Gunna Benjamin J. Mullenbachb May M. Poundstonea Jennifer B. Gordetskyc Edgar S. Underwooda Soroush Rais-Bahramid aDivision of Vascular and Interventional Radiology, Department of Radiology; Department of bRadiology, cPathology, and dUrology, University of Alabama at Birmingham, Birmingham, AL, USA Key Words Introduction Renal cell carcinoma • Embolization • Ablation • Interventional radiology Renal cell carcinoma (RCC) accounts for approxi- mately 4% of all cancer cases in the United States [1]. Abstract Its incidence, however, is increasing secondary to the This report describes the technical aspects of trans-arterial identification of renal tumors in patients who are im- embolization (TAE) of renal cell carcinoma prior to percuta- aged for other reasons [2–5]. One unintentional benefit neous ablation. All patients (n = 11) had a single renal mass of these incidentally-detected renal masses is that they (mean tumor diameter = 50.2 mm; range: 28–84 mm). Selec- are typically smaller, of lower grade, and have been as- tive TAE was performed via the common femoral artery. Em- sociated with longer disease-free survival [6]. Given bolic materials included: particles alone (n = 4), coils alone this smaller size, less invasive treatment options such (n = 1), particles + ethiodized oil (n = 2), particles + coils (n as partial nephrectomy, laparoscopic ablation, and per- = 1), ethiodized oil + ethanol (n = 2), and particles + ethanol cutaneous ablation are being used as safe and effective (n = 1). -

Traumatic Hemothorax Caused by Thoracic Wall and Intrathoracic
J Korean Soc Radiol 2021;82(4):923-935 Original Article https://doi.org/10.3348/jksr.2020.0116 eISSN 2288-2928 Received June 9, 2020 Revised August 8, 2020 Accepted August 11, 2020 Traumatic Hemothorax *Corresponding author Chang Ho Jeon, MD Department of Radiology, Biomedical Research Institute, Caused by Thoracic Wall and Pusan National University Hospital, 179 Gudeok-ro, Seo-gu, Busan 49241, Korea. Intrathoracic Injuries: Tel 82-51-240-7354 Fax 82-51-244-7534 E-mail [email protected] † Clinical Outcomes Current affiliation Department of Radiology, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic of Transcatheter Systemic University of Korea, Seoul, Korea. This is an Open Access article distributed under the terms of Artery Embolization the Creative Commons Attribu- tion Non-Commercial License 흉벽 및 흉곽 내 장기 손상으로 인한 외상성 혈흉: (https://creativecommons.org/ licenses/by-nc/4.0) which permits 전신 동맥 색전술의 임상 결과 unrestricted non-commercial use, distribution, and reproduc- tion in any medium, provided the 1 1,2 † 1 Chang Mu Lee, MD , Chang Ho Jeon, MD * , Rang Lee, MD , original work is properly cited. 1 1 3 Hoon Kwon, MD , Chang Won Kim, MD , Jin Hyeok Kim, MD , 4 4 4 Jae Hun Kim, MD , Hohyun Kim, MD , Seon Hee Kim, MD , ORCID iDs 4 5 6 Chang Mu Lee Chan Kyu Lee, MD , Chan Yong Park, MD , Miju Bae, MD https:// 1 2 4 Departments of Radiology, Biomedical Research Institute, Trauma and Surgical Critical Care, and orcid.org/0000-0001-6902-2919 6 Chang Ho Jeon Thoracic and Cardiovascular Surgery, Pusan National University Hospital, Busan, Korea 3 https:// Department of Radiology, Pusan National University Yangsan Hospital, Yangsan, Korea orcid.org/0000-0002-1762-5379 5 Department of Trauma Surgery, Wonkwang University Hospital, Iksan, Korea Rang Lee https:// orcid.org/0000-0001-9200-6466 Hoon Kwon https:// orcid.org/0000-0003-4055-5863 Purpose We aimed to assess the clinical efficacy of transcatheter arterial embolization (TAE) Chang Won Kim https:// for treating hemothorax caused by chest trauma.