Endovascular Treatment of Intracranial Aneurysms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spontaneous Hemothorax Caused by a Ruptured Intercostal Artery Aneurysm in Von Recklinghausen’S Neurofibromatosis
W.C. Chang, H.H. Hsu, H. Chang, et al BRIEF COMMUNICATION SPONTANEOUS HEMOTHORAX CAUSED BY A RUPTURED INTERCOSTAL ARTERY ANEURYSM IN VON RECKLINGHAUSEN’S NEUROFIBROMATOSIS Wei-Chou Chang,1 Hsian-He Hsu,1 Hung Chang,2 and Cheng-Yu Chen1 Abstract: Aneurysms arising from an intercostal artery are very rare vascular malformations in von Recklinghausen’s neurofibromatosis, which often have a silent clinical presentation and are difficult to diagnose before rupture. We report a case of von Recklinghausen’s neurofibromatosis with massive hemothroax caused by spontaneous rupture of an intercostal artery aneurysm in a 29-year-old man. The diagnosis was eventually confirmed by percutaneous angiography and treated with endovascular embolization. During a 10-month follow-up period, the patient had a satisfactory recovery. This case illustrates that angiography and possible endovascular embolization should be the first strategy in managing hemothorax in patients with von Recklinghausen’s disease. Key words: Aneurysm, ruptured; Embolization, therapeutic; Hemothorax; Neurofibromatosis 1; Thoracic arteries J Formos Med Assoc 2005;104:286-9 Von Recklinghausen’s neurofibromatosis, also named neurofibromatosis type 1 (NF-1), is a well-recognized Case Report entity that is characterized by numerous neuro- fibromas, spots of abnormal cutaneous pigmentation, A 29-year-old man presented at the emergency room and a variety of other dysplastic abnormalities of with sudden onset shortness of breath and severe the skin, nervous system, bones, endocrine organs and retrosternal pain radiating to his back. One week prior blood vessels.1,2 Vascular abnormalities in patients to admission, dull discomfort upon breathing was with NF-1 have been described for large, medium- noted, which was not affected by position or physical sized and small muscular arteries in the intracranial, activity. -

Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: a Preliminary Multiscale Numerical Investigation
applied sciences Article Impacts of Internal Carotid Artery Revascularization on Flow in Anterior Communicating Artery Aneurysm: A Preliminary Multiscale Numerical Investigation Guang-Yu Zhu 1, Yuan Wei 1, Ya-Li Su 2, Qi Yuan 1 and Cheng-Fu Yang 3,* 1 School of Energy and Power Engineering, Xi’an Jiaotong University, Xi’an 710049, China; [email protected] (G.-Y.Z.); [email protected] (Y.W.); [email protected] (Q.Y.) 2 School of Mechanical Engineering, Xi’an Shiyou University, Xi’an 710065, China; [email protected] 3 Department of Chemical and Materials Engineering, National University of Kaohsiung, No. 700, Kaohsiung University Rd., Nan-Tzu District, Kaohsiung 811, Taiwan * Correspondence: [email protected] Received: 5 September 2019; Accepted: 26 September 2019; Published: 3 October 2019 Abstract: The optimal management strategy of patients with concomitant anterior communicating artery aneurysm (ACoAA) and internal carotid artery (ICA) stenosis is unclear. This study aims to evaluate the impacts of unilateral ICA revascularization on hemodynamics factors associated with rupture in an ACoAA. In the present study, a multiscale computational model of ACoAA was developed by coupling zero-dimensional (0D) models of the cerebral vascular system with a three-dimensional (3D) patient-specific ACoAA model. Distributions of flow patterns, wall shear stress (WSS), relative residence time (RRT) and oscillating shear index (OSI) in the ACoAA under left ICA revascularization procedure were quantitatively assessed by using transient computational fluid dynamics (CFD) simulations. Our results showed that the revascularization procedures significantly changed the hemodynamic environments in the ACoAA. The flow disturbance in the ACoAA was enhanced by the resumed flow from the affected side. -

The Surgical Management of Pituitary Apoplexy with Occluded Internal Carotid Artery and Hidden Intracranial Aneurysm: Illustrative Case
J Neurosurg Case Lessons 2(5):CASE20115, 2021 DOI: 10.3171/CASE20115 The surgical management of pituitary apoplexy with occluded internal carotid artery and hidden intracranial aneurysm: illustrative case *Jian-Dong Zhu, MD, Sungel Xie, MD, Ling Xu, MD, Ming-Xiang Xie, MD, and Shun-Wu Xiao, MD Department of Neurosurgery, Affiliated Hospital of Zunyi Medical University, Guizhou, China BACKGROUND Approximately 0.6% to 12% of cases of pituitary adenoma are complicated by apoplexy, and nearly 6% of pituitary adenomas are comorbid aneurysms. Occlusion of the internal carotid artery (ICA) with hidden intracranial aneurysm due to compression by an apoplectic pituitary adenoma is extremely rare; thus, the surgical strategy is also unknown. OBSERVATIONS The authors reported the case of a 48-year-old man with a large pituitary adenoma with coexisting ICA occlusion. After endoscopic transnasal surgery, repeated computed tomography angiography (CTA) demonstrated reperfusion of the left ICA but with a new-found aneurysm in the left posterior communicating artery; thus, interventional aneurysm embolization was performed. With stable recovery and improved neurological condition, the patient was discharged for rehabilitation training. LESSONS For patients with pituitary apoplexy accompanied by a rapid decrease of neurological conditions, emergency decompression through endoscopic endonasal transsphenoidal resection can achieve satisfactory results. However, with occlusion of the ICA by enlarged pituitary adenoma or pituitary apoplexy, a hidden but rare intracranial aneurysm may be considered when patients are at high risk of such vascular disease as aneurysm, and gentle intraoperative manipulations are required. Performing CTA or digital subtraction angiography before and after surgery can effectively reduce the missed diagnosis of comorbidity and thus avoid life-threatening bleeding events from the accidental rupture of an aneurysm. -

Relationship Between Cerebrovascular Atherosclerotic Stenosis and Rupture Risk of Unruptured Intracranial Aneurysm a Single-Cen
Clinical Neurology and Neurosurgery 186 (2019) 105543 Contents lists available at ScienceDirect Clinical Neurology and Neurosurgery journal homepage: www.elsevier.com/locate/clineuro Relationship between cerebrovascular atherosclerotic stenosis and rupture risk of unruptured intracranial aneurysm: A single-center retrospective T study Xin Fenga,b, Peng Qia, Lijun Wanga, Jun Lua, Hai Feng Wanga, Junjie Wanga, Shen Hua, Daming Wanga,b,⁎ a Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, No. 1 DaHua Road, Dong Dan, Beijing, 100730, China b Graduate School of Peking Union Medical College, No. 9 Dongdansantiao, Dongcheng District, Beijing, 100730, China ARTICLE INFO ABSTRACT Keywords: Objectives: Cerebrovascular atherosclerotic stenosis (CAS) and intracranial aneurysm (IA) have a common un- Atherosclerotic stenosis derlying arterial pathology and common risk factors, but the clinical significance of CAS in IA rupture (IAR) is Intracranial aneurysm unclear. This study aimed to investigate the effect of CAS on the risk of IAR. Risk factor Patients and methods: A total of 336 patients with 507 sacular IAs admitted at our center were included. Rupture Univariable and multivariable logistic regression analyses were performed to determine the association between IAR and the angiographic variables for CAS. We also explored the differences in CAS in patients aged < 65 and ≥65 years. Results: In all the patient groups, moderate (50%–70%) cerebrovascular stenosis was significantly associated with IAR (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.8–6.5). Single cerebral artery stenosis was also significantly associated with IAR (OR, 2.3; 95% CI, 1.3–3.9), and intracranial stenosis may be a risk factor for IAR (OR, 1.8; 95% CI, 1.0–3.2). -

SUBARACHNOID HAEMORRHAGE and INTRACRANIAL ANEURYSMS: WHAT NEUROLOGISTS NEED to KNOW I28* P J Kirkpatrick
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.73.suppl_1.i28 on 1 September 2002. Downloaded from SUBARACHNOID HAEMORRHAGE AND INTRACRANIAL ANEURYSMS: WHAT NEUROLOGISTS NEED TO KNOW i28* P J Kirkpatrick J Neurol Neurosurg Psychiatry 2002;73(Suppl I):i28–i33 he incidence of stroke caused by subarachnoid haemorrhage (SAH) remains constant, with intracranial aneurysm rupture causing SAH in up to 5000 patients in the UK per annum. TAlthough this represents less than 5% of all strokes, recognition is of crucial importance since intervention can radically alter outcome. The combined mortality and morbidity for aneurysm rupture reaches 50%; since the condition can affect individuals at any age, long term morbidity in survivors can be substantial.1 Failure to diagnose SAH exposes a patient to the fatal effects of a fur- ther bleed, and also to complications which can now be avoided or successfully treated.23 cPATHOLOGY SAH refers to a leakage of blood into the subarachnoid spaces (fig 1A) which is a continuous space between the supratentorial and infratentorial compartments. A greater concentration of blood products around the site of the bleed is usual, but SAH originating from a focal source can be more diffuse and spread throughout wider aspects of the subarachnoid space. Haemorrhage can extend into adjacent parenchymal structures (fig 1B) and ventricular system, with associated high morbidity and mortality (fig 1C). Inflammatory processes (table 1), excited by the presence of red cell breakdown products, affect copyright. the large vessels of the circle of Willis and smaller vessels within the subpial space.4 These processes are complex, but combine to impair the adequate distribution of blood to affected territories. -
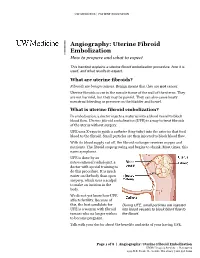
Uterine Fibroid Embolization Procedure, How It Is Used, and What Results to Expect
UW MEDICINE | PATIENT EDUCATION | | Angiography: Uterine Fibroid | Embolization | How to prepare and what to expect This handout explains a uterine fibroid embolization procedure, how it is used, and what results to expect. What are uterine fibroids? Fibroids are benign tumors. Benign means that they are not cancer. Uterine fibroids occur in the muscle tissue of the wall of the uterus. They are not harmful, but they may be painful. They can also cause heavy menstrual bleeding or pressure on the bladder and bowel. What is uterine fibroid embolization? In embolization, a doctor injects a material into a blood vessel to block blood flow. Uterine fibroid embolization (UFE) is a way to treat fibroids DRAFTof the uterus without surgery. UFE uses X-rays to guide a catheter (tiny tube) into the arteries that feed blood to the fibroid. Small particles are then injected to block blood flow. With its blood supply cut off, the fibroid no longer receives oxygen and nutrients. The fibroid stops growing and begins to shrink. Most times, this eases symptoms. UFE is done by an interventional radiologist, a doctor with special training to do this procedure. It is much easier on the body than open surgery, which uses a scalpel to make an incision in the body. We do not yet know how UFE affects fertility. Because of this, the best candidate for During UFE, small particles are injected UFE is a woman with fibroid into blood vessels to block blood flow to tumors who no longer wishes the fibroid. to become pregnant. Talk with your doctor about the benefits and risks of your having UFE. -

Morphological Variables Associated with Ruptured Basilar Tip Aneurysms Jian Zhang1,2,12, Anil Can1,3,12, Pui Man Rosalind Lai1, Srinivasan Mukundan Jr.4, Victor M
www.nature.com/scientificreports OPEN Morphological variables associated with ruptured basilar tip aneurysms Jian Zhang1,2,12, Anil Can1,3,12, Pui Man Rosalind Lai1, Srinivasan Mukundan Jr.4, Victor M. Castro5, Dmitriy Dligach6,7, Sean Finan6, Vivian S. Gainer5, Nancy A. Shadick8, Guergana Savova6, Shawn N. Murphy5,9, Tianxi Cai10, Scott T. Weiss8,11 & Rose Du1,11* Morphological factors of intracranial aneurysms and the surrounding vasculature could afect aneurysm rupture risk in a location specifc manner. Our goal was to identify image-based morphological parameters that correlated with ruptured basilar tip aneurysms. Three-dimensional morphological parameters obtained from CT-angiography (CTA) or digital subtraction angiography (DSA) from 200 patients with basilar tip aneurysms diagnosed at the Brigham and Women’s Hospital and Massachusetts General Hospital between 1990 and 2016 were evaluated. We examined aneurysm wall irregularity, the presence of daughter domes, hypoplastic, aplastic or fetal PCoAs, vertebral dominance, maximum height, perpendicular height, width, neck diameter, aspect and size ratio, height/width ratio, and diameters and angles of surrounding parent and daughter vessels. Univariable and multivariable statistical analyses were performed to determine statistical signifcance. In multivariable analysis, presence of a daughter dome, aspect ratio, and larger fow angle were signifcantly associated with rupture status. We also introduced two new variables, diameter size ratio and parent-daughter angle ratio, which were both signifcantly inversely associated with ruptured basilar tip aneurysms. Notably, multivariable analyses also showed that larger diameter size ratio was associated with higher Hunt-Hess score while smaller fow angle was associated with higher Fisher grade. These easily measurable parameters, including a new parameter that is unlikely to be afected by the formation of the aneurysm, could aid in screening strategies in high-risk patients with basilar tip aneurysms. -

The Genetics of Intracranial Aneurysms
J Hum Genet (2006) 51:587–594 DOI 10.1007/s10038-006-0407-4 MINIREVIEW Boris Krischek Æ Ituro Inoue The genetics of intracranial aneurysms Received: 20 February 2006 / Accepted: 24 March 2006 / Published online: 31 May 2006 Ó The Japan Society of Human Genetics and Springer-Verlag 2006 Abstract The rupture of an intracranial aneurysm (IA) neurovascular diseases. Its most frequent cause is the leads to a subarachnoid hemorrhage, a sudden onset rupture of an intracranial aneurysm (IA), which is an disease that can lead to severe disability and death. Sev- outpouching or sac-like widening of a cerebral artery. eral risk factors such as smoking, hypertension and Initial diagnosis is usually evident on a cranial computer excessive alcohol intake are associated with subarachnoid tomography (CT) showing extravasated (hyperdense) hemorrhage. IAs, ruptured or unruptured, can be treated blood in the subarachnoid space. In a second step, the either surgically via a craniotomy (through an opening in gold standard of diagnostic techniques to detect the the skull) or endovascularly by placing coils through a possible underlying ruptured aneurysm is intra-arterial catheter in the femoral artery. Even though the etiology digital subtraction angiography and additional three- of IA formation is mostly unknown, several studies dimensional (3D) rotational angiography (panels A and support a certain role of genetic factors. In reports so far, B in Fig. 1). Recently non-invasive diagnostic imaging genome-wide linkage studies suggest several susceptibil- modalities are becoming increasingly sophisticated. 3D ity loci that may contain one or more predisposing genes. CT angiography and 3D magnetic resonance angiogra- Studies of several candidate genes report association with phy allow less invasive methods to reliably depict IAs IAs. -

Embolization of Brain Aneurysms and Arteriovenous
Embolization of Brain Aneurysms and Arteriovenous Malformations/Fistulas Embolization of brain aneurysms and arteriovenous malformations (AVM) uses imaging guidance to place small, soft metal coils into an aneurysm to block the flow of blood and prevent the aneurysm from rupturing. It also is used to fill AVMs – abnormal connections between arteries and veins – with liquid embolic agents (similar to fast-sealing glue). AVMs may prevent oxygenated blood from completely circulating throughout the brain and can cause a variety of problems, including headache, weakness, and other neurological symptoms. Embolization treats cerebral aneurysms and AVMs previously thought inoperable and is much less invasive than open surgery. Your doctor will instruct you on how to prepare, including any changes to your medication schedule. Tell your doctor if there's a possibility you are pregnant and discuss any recent illnesses, medical conditions, allergies and medications you're taking, including herbal supplements and aspirin. You may be advised to stop taking aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) or blood thinners several days prior to your procedure. You also may be told not to eat or drink anything after midnight before your procedure. Plan to stay at the hospital overnight. Leave jewelry at home and wear loose, comfortable clothing. You will be asked to wear a gown. What is Embolization of Brain Aneurysms and Fistulas? Embolization of brain aneurysms and arteriovenous malformations (AVM)/fistulas is a minimally invasive treatment for aneurysms and other blood vessel malformations that occur in the brain. These problems are typically identified in adults; however, aneurysms and AVMs can also occur in children. -

The Biophysical Role of Hemodynamics in the Pathogenesis of Cerebral Aneurysm Formation and Rupture
NEUROSURGICAL FOCUS Neurosurg Focus 47 (1):E11, 2019 The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture Sauson Soldozy, BA, Pedro Norat, MD, Mazin Elsarrag, MS, Ajay Chatrath, MS, John S. Costello, BA, Jennifer D. Sokolowski, MD, PhD, Petr Tvrdik, PhD, M. Yashar S. Kalani, MD, PhD, and Min S. Park, MD Department of Neurological Surgery, University of Virginia Health System, Charlottesville, Virginia The pathogenesis of intracranial aneurysms remains complex and multifactorial. While vascular, genetic, and epidemio- logical factors play a role, nascent aneurysm formation is believed to be induced by hemodynamic forces. Hemodynamic stresses and vascular insults lead to additional aneurysm and vessel remodeling. Advanced imaging techniques allow us to better define the roles of aneurysm and vessel morphology and hemodynamic parameters, such as wall shear stress, oscillatory shear index, and patterns of flow on aneurysm formation, growth, and rupture. While a complete understand- ing of the interplay between these hemodynamic variables remains elusive, the authors review the efforts that have been made over the past several decades in an attempt to elucidate the physical and biological interactions that govern aneurysm pathophysiology. Furthermore, the current clinical utility of hemodynamics in predicting aneurysm rupture is discussed. https://thejns.org/doi/abs/10.3171/2019.4.FOCUS19232 KEYWORDS cerebral aneurysm; hemodynamics; wall shear stress; computational fluid dynamics; vascular remodeling NTRACRANIAL aneurysms (IAs) are acquired outpouch- cades and, ultimately, a wide range of transcriptional and ings of arteries that occur in 1%–2% of the popula- signaling changes that lead to vascular wall remodeling. tion.36 Likely as a result of improved imaging modali- The advent of computational and radiographic modeling Ities, the incidence of unruptured IAs has increased. -

Pulmonary Embolism A-Nd Thrombosis R
Postgrad Med J: first published as 10.1136/pgmj.38.435.13 on 1 January 1962. Downloaded from POSTGRAD MED. J. (I962), 38, 13 PULMONARY EMBOLISM A-ND THROMBOSIS R. MARSHALL, M.D., M.R.C.P. Nuffield Department of Surgery, Radcliffe Infirmary, Oxford SUDDEN death following a call for a bedpan is a chest pain and ECG changes when the balloon classical mode of presentation of pulmonary em- was inflated in a smaller artery (Brofman, Charms, bolism, but it is probable that cases of this severity Kohn, Elder, Newman and Rizika, I957). On the form only a small proportion of the total. Pul- other hand, it is a common practice to impact the monary embolism may be caused by blood clot, tip of a cardiac catheter in branches of the pul- fat, cells or foreign bodies, but most commonly by monary artery of about 2 mm. diameter with no blood clot. ill effects. In spite of the undoubted ability of emboli to produce reflex effects under certain cir- cumstances, there is a growing body of evidence The Physiological Effects that the effects of pulmonary embolism by inert The physiological effects ofpulmonary embolism particles are produced by the magnitude of the depend upon the size and number of the emboli mechanical blockage of the pulmonary vessels and and they may produce these effects either by are not dependent upon the size of the emboli or mechanical blockage of the circulation when they reflex vasoconstriction (Daley, I957; McEvoy, are of sufficient size or number, or by the initiation Harder and Dale, 1958; Williams, 1956). -

Ruptured Aneurysm–Induced Pituitary Apoplexy: Illustrative Case
J Neurosurg Case Lessons 1(26):CASE21169, 2021 DOI: 10.3171/CASE21169 Ruptured aneurysm–induced pituitary apoplexy: illustrative case Michiharu Yoshida, MD, PhD,1 Takeshi Hiu, MD, PhD,1 Shiro Baba, MD, PhD,1 Minoru Morikawa, MD, PhD,2 Nobutaka Horie, MD, PhD,1 Kenta Ujifuku, MD, PhD,1 Koichi Yoshida, MD, PhD,1 Yuki Matsunaga, MD,1 Daisuke Niino, MD, PhD,3 Ang Xie, BS,1 Tsuyoshi Izumo, MD, PhD,1 Takeo Anda, MD, PhD,1 and Takayuki Matsuo, MD, PhD1 Departments of 1Neurosurgery, 2Radiology, and 3Pathology, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan BACKGROUND Pituitary apoplexy associated with aneurysmal rupture is extremely rare and may be misdiagnosed as primary pituitary adenoma apoplexy. The authors present a case of a patient with pituitary apoplexy caused by rupture of an anterior cerebral artery aneurysm embedded within a giant pituitary adenoma, and they review the relevant literature. OBSERVATIONS A 78-year-old man experienced sudden headache with progressive vision loss. Magnetic resonance imaging (MRI) revealed a giant pituitary tumor with abnormal signal intensity. Magnetic resonance angiography immediately before surgery showed a right A1 segment aneurysm, suggesting coexisting pituitary apoplexy and ruptured aneurysm. The patient underwent urgent transsphenoidal surgery for pituitary apoplexy. The tumor was partially removed, but the perianeurysmal component was left behind. Subsequent cerebral angiography showed a 5-mm right A1 aneurysm with a bleb that was successfully embolized with coils. Retrospective review of preoperative dynamic MRI showed extravasation of contrast medium from the ruptured aneurysm into the pituitary adenoma. Histopathologic examination showed gonadotroph adenoma with hemorrhagic necrosis.