5 Combinations of Hypoxia-Targeting Compounds and Radiation-Activated Prodrugs with Ionizing Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

UCSD Moores Cancer Center Neuro-Oncology Program
UCSD Moores Cancer Center Neuro-Oncology Program Recent Progress in Brain Tumors 6DQWRVK.HVDUL0'3K' 'LUHFWRU1HXUR2QFRORJ\ 3URIHVVRURI1HXURVFLHQFHV 0RRUHV&DQFHU&HQWHU 8QLYHUVLW\RI&DOLIRUQLD6DQ'LHJR “Brain Cancer for Life” Juvenile Pilocytic Astrocytoma Metastatic Brain Cancer Glioblastoma Multiforme Glioblastoma Multiforme Desmoplastic Infantile Ganglioglioma Desmoplastic Variant Astrocytoma Medulloblastoma Atypical Teratoid Rhabdoid Tumor Diffuse Intrinsic Pontine Glioma -Mutational analysis, microarray expression, epigenetic phenomenology -Age-specific biology of brain cancer -Is there an overlap? ? Neuroimmunology ? Stem cell hypothesis Courtesy of Dr. John Crawford Late Effects Long term effect of chemotherapy and radiation on neurocognition Risks of secondary malignancy secondary to chemotherapy and/or radiation Neurovascular long term effects: stroke, moya moya Courtesy of Dr. John Crawford Importance Increase in aging population with increased incidence of cancer Patients with cancer living longer and developing neurologic disorders due to nervous system relapse or toxicity from treatments Overview Introduction Clinical Presentation Primary Brain Tumors Metastatic Brain Tumors Leptomeningeal Metastases Primary CNS Lymphoma Paraneoplastic Syndromes Classification of Brain Tumors Tumors of Neuroepithelial Tissue Glial tumors (astrocytic, oligodendroglial, mixed) Neuronal and mixed neuronal-glial tumors Neuroblastic tumors Pineal parenchymal tumors Embryonal tumors Tumors of Peripheral Nerves Shwannoma Neurofibroma -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

The Oxygen Effect in the Development of Radiosensitizers
Translational Oncology Volume 4 Number 4 August 2011 pp. 189–198 189 www.transonc.com Six Degrees of Separation: Bryan T. Oronsky*, Susan J. Knox† and Jan Scicinski* The Oxygen Effect *RadioRx, Inc, Mountain View, CA, USA; †Department in the Development of Radiation Oncology, Stanford University Medical of Radiosensitizers Center, Stanford, CA, USA Abstract The popular theory six degrees of separation is used in this review as an analogy to relate all radiosensitization to oxygen. As the prime mover of all radiosensitizers, the pervasive influence of oxygen has consciously or uncon- sciously influenced the direction of research and development and provided the benchmark against which all other compounds and approaches are measured. It is the aim of this review to develop the six degrees of separation from oxygen analogy as a unifying framework for conceptually organizing the field and for giving context to its varied subspecializations and theories. Under such a framework, it would become possible for one area to consider ques- tions and problems found in other areas of radiosensitization, using a common analogy, that would allow for further development and unification of this multifaceted discipline. In this review, approaches to the development of radio- sensitizers and the current state of research in this field are discussed, including promising new agents in various stages of clinical development. Translational Oncology (2011) 4, 189–198 Introduction support metabolic pathways. The ability of more aggressive cancer The view that everything is connected to everything else according to cells to survive hypoxic conditions leads to selection against apoptosis the popular six degrees of separation theory finds corroboration on a and an increased resistance to chemotherapy, as well as a propensity therapeutic level in the field of radiosensitization. -

Repeated Tumor Oximetry to Identify Therapeutic Window During Metronomic Cyclophosphamide Treatment of 9L Gliomas
ONCOLOGY REPORTS 26: 281-286, 2011 Repeated tumor oximetry to identify therapeutic window during metronomic cyclophosphamide treatment of 9L gliomas SRIRAM MUPPARAJU1,2, HUAGANG HOU1,2, JEAN P. LARIVIERE1, HAROLD SwaRTZ1,2, YOUSSEF JOUNAIDI3 and NADEEM KHAN1,2 1EPR Center for Viable Systems, Dartmouth Medical School, Hanover, NH 03755; 2Norris Cotton Cancer Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756; 3Division of Cell and Molecular Biology, Department of Biology, Boston University, Boston, MA 02215, USA Received February 1, 2011; Accepted March 17, 2011 DOI: 10.3892/or.2011.1268 Abstract. Malignant gliomas are aggressive and angiogenic Introduction tumors with high VEGF content. Consequently, approaches such as metronomic chemotherapy, which have an anti- Gliomas are highly angiogenic tumors with rapid infiltrative angiogenic effect, are being investigated. However, a lack of growth and profound microvascular proliferation (1). Despite an appropriate technique that can facilitate the identification improvements in surgery and radiotherapy; the prognosis of vascular changes during antiangiogenic treatments has of glioma patients has remained poor (2,3). Consequently, restricted therapeutic optimization. We have investigated the new methods are urgently needed to improve and synergize potential of tumor pO2 as a marker to detect vascular changes strategies for the treatment of gliomas. The presence of tumor during metronomic chemotherapy. Electron paramagnetic hypoxia (pO2 <10-15 mmHg) further compromises the outcome resonance (EPR) oximetry was used to repeatedly assess tumor by stimulating glioma progression, aggressive phenotypes, pO2 during metronomic cyclophosphamide treatment of subcu- metastases and resistance to therapies (4-6). Tumor hypoxia taneous 9L tumors. The 9L tumors were hypoxic with a pO2 of cannot be predicted by tumor type or size and therefore must 5.6-8 mmHg and a tumor volume of 247-300 mm3 prior to any be measured. -

L:\0901 with Peru\0901PHARMAPPX.Wpd
Harmonized Tariff Schedule of the United States (2009) (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2009) (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABAPERIDONE 183849-43-6 ACITAZANOLAST 114607-46-4 ABARELIX 183552-38-7 ACITEMATE 101197-99-3 ABATACEPT 332348-12-6 ACITRETIN 55079-83-9 ABCIXIMAB 143653-53-6 ACIVICIN 42228-92-2 ABECARNIL 111841-85-1 ACLANTATE 39633-62-0 ABETIMUS 167362-48-3 ACLARUBICIN 57576-44-0 ABIRATERONE 154229-19-3 ACLATONIUM NAPADISILATE 55077-30-0 ABITESARTAN 137882-98-5 ACODAZOLE 79152-85-5 ABLUKAST 96566-25-5 ACOLBIFENE 182167-02-8 ABRINEURIN 178535-93-8 ACONIAZIDE 13410-86-1 ABUNIDAZOLE 91017-58-2 ACOTIAMIDE 185106-16-5 ACADESINE 2627-69-2 ACOXATRINE 748-44-7 ACAMPROSATE 77337-76-9 ACREOZAST 123548-56-1 ACAPRAZINE 55485-20-6 -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

I Regulations
23.2.2007 EN Official Journal of the European Union L 56/1 I (Acts adopted under the EC Treaty/Euratom Treaty whose publication is obligatory) REGULATIONS COUNCIL REGULATION (EC) No 129/2007 of 12 February 2007 providing for duty-free treatment for specified pharmaceutical active ingredients bearing an ‘international non-proprietary name’ (INN) from the World Health Organisation and specified products used for the manufacture of finished pharmaceuticals and amending Annex I to Regulation (EEC) No 2658/87 THE COUNCIL OF THE EUROPEAN UNION, (4) In the course of three such reviews it was concluded that a certain number of additional INNs and intermediates used for production and manufacture of finished pharmaceu- ticals should be granted duty-free treatment, that certain of Having regard to the Treaty establishing the European Commu- these intermediates should be transferred to the list of INNs, nity, and in particular Article 133 thereof, and that the list of specified prefixes and suffixes for salts, esters or hydrates of INNs should be expanded. Having regard to the proposal from the Commission, (5) Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff (1) established the Combined Nomenclature Whereas: (CN) and set out the conventional duty rates of the Common Customs Tariff. (1) In the course of the Uruguay Round negotiations, the Community and a number of countries agreed that duty- (6) Regulation (EEC) No 2658/87 should therefore be amended free treatment should be granted to pharmaceutical accordingly, products falling within the Harmonised System (HS) Chapter 30 and HS headings 2936, 2937, 2939 and 2941 as well as to designated pharmaceutical active HAS ADOPTED THIS REGULATION: ingredients bearing an ‘international non-proprietary name’ (INN) from the World Health Organisation, specified salts, esters or hydrates of such INNs, and designated inter- Article 1 mediates used for the production and manufacture of finished products. -
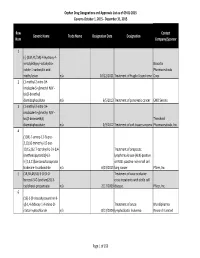
Orphan Drug Designation List
Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 (‐)‐(3aR,4S,7aR)‐4‐Hydroxy‐4‐ m‐tolylethynyl‐octahydro‐ Novartis indole‐1‐carboxylic acid Pharmaceuticals methyl ester n/a 10/12/2011 Treatment of Fragile X syndrome Corp. 2 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐broethyl) diamidophosphate n/a 6/5/2013 Treatment of pancreatic cancer EMD Serono 3 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐bromoethyl) Threshold diamidophosphate n/a 3/9/2012 Treatment of soft tissue sarcoma Pharmaceuticals, Inc. 4 (1OR)‐7‐amino‐12‐fluoro‐ 2,10,16‐trimethyl‐15 oxo‐ 10,15,16,17‐tetrahydro‐2H‐8,4‐ Treatment of anaplastic (metheno)pyrazolo[4,3‐ lymphoma kinase (ALK)‐positive h][2,5,11]benzoxadiazacyclote or ROS1‐positive non‐small cell tradecine‐3‐carbonitrile n/a 6/23/2015 lung cancer Pfizer, Inc. 5 (1R,3R,4R,5S)‐3‐O‐[2‐O‐ Treatment of vaso‐occlusive benzoyl‐3‐O‐(sodium(2S)‐3‐ crisis in patients with sickle cell cyclohexyl‐propanoate‐ n/a 2/17/2009 disease. Pfizer, Inc. 6 (1S)‐1‐(9‐deazahypoxanthin‐9‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ Treatment of acute Mundipharma ribitol‐hydrochloride n/a 8/13/2004 lymphoblastic leukemia Research Limited Page 1 of 359 Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 7 Treatment of chronic lymphocytic leukemia and related leukemias to include (1S)‐1‐(9‐deazahypoxanthin‐9‐ prolymphocytic leukemia, adult T‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ cell leukemia, and hairy cell Mundipharma ribitol‐hydrochloride n/a 8/10/2004 leukemia Research Ltd. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0226431 A1 Habib (43) Pub
US 20090226431A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0226431 A1 Habib (43) Pub. Date: Sep. 10, 2009 (54) TREATMENT OF CANCER AND OTHER Publication Classification DISEASES (51) Int. Cl. A 6LX 3/575 (2006.01) (76)76) InventorInventor: Nabilabil Habib,Habib. Beirut (LB(LB) C07J 9/00 (2006.01) Correspondence Address: A 6LX 39/395 (2006.01) 101 FEDERAL STREET A6IP 29/00 (2006.01) A6IP35/00 (2006.01) (21) Appl. No.: 12/085,892 A6IP37/00 (2006.01) 1-1. (52) U.S. Cl. ...................... 424/133.1:552/551; 514/182: (22) PCT Filed: Nov.30, 2006 514/171 (86). PCT No.: PCT/US2O06/045665 (57) ABSTRACT .."St. Mar. 6, 2009 The present invention relates to a novel compound (e.g., 24-ethyl-cholestane-3B.5C,6C.-triol), its production, its use, and to methods of treating neoplasms and other tumors as Related U.S. Application Data well as other diseases including hypercholesterolemia, (60) Provisional application No. 60/741,725, filed on Dec. autoimmune diseases, viral diseases (e.g., hepatitis B, hepa 2, 2005. titis C, or HIV), and diabetes. F2: . - 2 . : F2z "..., . Cz: ".. .. 2. , tie - . 2 2. , "Sphagoshgelin , , re Cls Phosphatidiglethanolamine * - 2 .- . t - r y ... CBs .. A . - . Patent Application Publication Sep. 10, 2009 Sheet 1 of 16 US 2009/0226431 A1 E. e'' . Phosphatidylcholine. " . Ez'.. C.2 . Phosphatidylserias. * . - A. z' C. w E. a...2 .". is 2 - - " - B 2. Sphingoshgelin . Cls Phosphatidglethanglamine Figure 1 Patent Application Publication Sep. 10, 2009 Sheet 2 of 16 US 2009/0226431 A1 Chile Phosphater Glycerol Phosphatidylcholine E. -

Orphan Drug Dummy File
Orphan Drug Designations and Approvals List as of 09‐01‐2016 Governs October 1, 2016 ‐ December 31, 2016 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 1. Prevention of secondary carnitine deficiency in valproic acid toxicity 2. Treatment of secondary carnitine deficiency in Sigma-Tau levocarnitine Carnitor 11/15/1989 valproic acid toxicity Pharmaceuticals, Inc. 2 1. Treatment of graft versus host disease in patients receiving bone marrow transplantation 2. Prevention of graft versus host disease in patients receiving Pediatric thalidomide n/a 9/19/1988 bone marrow transplantation Pharmaceuticals, Inc. 3 A Diagnostic for the management Advanced Imaging Theranost 68 Ga RGD n/a 10/1/2014 of Moyamoya disease (MMD) Projects, LLC (AIP) 4 Cadila heat killed Mycobacterium w Pharmaceuticals immunomodulator Cadi Mw 9/3/2004 Active tuberculosis Limited, Inc. 5 Adjunct to cytokine therapy in the treatment of acute myeloid Histamine Ceplene 12/15/1999 leukemia. EpiCept Corporation 6 Adjunct to surgery in cases of rh-microplasmin, ocriplasmin Jetrea 3/16/2004 pediatric vitrectomy ThromboGenics Inc. 7 Adjunct to the non-operative management of secreting cutaneous fistulas of the stomach, duodenum, small intestine (jejunum and ileum), or Ferring Laboratories, Somatostatin Zecnil 6/20/1988 pancreas. Inc. Page 1 of 377 Orphan Drug Designations and Approvals List as of 09‐01‐2016 Governs October 1, 2016 ‐ December 31, 2016 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 8 Adjunct to whole brain radiation therapy for the treatment of brain metastases in patients with Allos Therapeutics, efaproxiral n/a 7/28/2004 breast cancer Inc. -

PHRM 203 Allison Beale Overview
PHRM 203 Allison Beale Overview • Neoplasms – Introduction • Chemotherapy often – Causes associated with: – Types – Secondary malignancies – Seizures (~13% of patients) • Antineoplastic agents – Nausea & vomiting – Introduction – Examples A Beale PHRM 203 - Antineoplastic Agents 2 Neoplasms Introduction TERMS – Anaplasia • 2nd leading cause of • Loss of cellular death in US after CV organization • All cancers start with a – Autonomy cell or cells that is • Ignore growth regulations genetically different from the surrounding – Metastasis cells; all cell types can • Spread into other tissues become cancerous – Angiogenesis • Create their own blood supply A Beale PHRM 203 - Antineoplastic Agents 3 Neoplasms Causes • Genetic predisposition – Li-Fraumeni Syndrome – Familial Adenomatous Polyposis • Viral infection (e.g., herpes, HPV, EBV, HBV) • Nematode infection (e.g., Spirocerca lupi) • Constant irritation or inflammation • Stress • Chemicals (mutagens, carcinogens, e.g., Cisplatin) • Radiation (uv, ionizing) A Beale PHRM 203 - Antineoplastic Agents 4 Neoplasms • Solid Types – Carcinomas • Tumors of epithelium – Adenomas versus adenocarcinomas – Melanoma versus malignant melanoma (melanocarcinoma) – Sarcomas • Tumors of mesenchymal origin Osteoblasts in – Fibroma versus fibrosarcoma osteomas – Lymphoma versus lymphosarcoma produce LOTS of PG-E; pain – Osteoma versus osteosarcoma controlled with • Hematological malignancies ASPIRIN! – Leukemia (general term for cancer of white blood cells) A Beale PHRM 203 - Antineoplastic Agents 5 Carcinomas