Mendelevium and Rutherfordium 10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Hobart Mendeleev 2-3-11.Pdf
Czar Nicholas II and Alexi at Tobolsk, Siberia in 1917 - Beinecke Library, Yale University Dr. David Hobart Los Alamos National Laboratory Academician Boris Myasoedov Secretary General Russian Academy of Sciences U N C L A S S I F I E D LA-UR-09-05702 U N C L A S S I F I E D National Laboratory From Modest Beginnings Dmitri Ivanovich Mendeleev was born on February 8th, 1834 in Verhnie Aremzyani near Tobolsk, Russian Empire From modest beginnings in a small village in Siberia an extraordinary Russian chemist conceived of a profound and revolutionary scientific contribution to modern science: the Periodic Table of the Elements. The Greek Periodic Table ~ 400 BC As with most profound discoveries a number of important developments and observations were made prior to that discovery Definition of an Element In 1661 Boyle criticized the experiments of “alchemists” - Chemistry is the science of the composition of substances - not merely an adjunct to the art of alchemy - Elements are the un-decomposable constituents of material bodies - Understanding the distinction between mixtures and compounds, he made progress in detecting their ingredients - which he termed analysis Robert Boyle 1627-1691 The Atomic Theory The Greek philosopher Democritus first proposed the atomic theory but centuries later John Dalton established the scientific foundation: - All atoms of a given element are identical - The atoms of different elements can be distinguished by their relative weights Democritus of Abdera John Dalton 460-370 BC 1766-1844 Early Knowledge and Discovery The elements gold, silver, copper, tin, Searching for the “Philosopher’s lead, mercury, and others were known Stone” German alchemist Henning from antiquity. -

Genius of the Periodic Table
GENIUS OF THE PERIODIC TABLE "Isn't it the work of a genius'. " exclaimed Academician V.I. Spitsyn, USSR, a member of the Scientific Advisory Committee when talking to an Agency audience in January. His listeners shared his enthusiasm. Academician Spitsyn was referring to the to the first formulation a hundred years ago by Professor Dmitry I. Mendeleyev of the Periodic Law of Elements. In conditions of enormous difficulty, considering the lack of data on atomic weights of elements, Mendeleyev created in less than two years work at St. Petersburg University, a system of chemical elements that is, in general, still being used. His law became a powerful instrument for further development of chemistry and physics. He was able immediately to correct the atomic weight numbers of some elements, including uranium, whose atomic weight he found to be double that given at the time. Two years later Mendeleyev went so far as to give a detailed description of physical or chemical properties of some elements which were as yet undiscovered. Time gave striking proof of his predictions and his periodic law. Mendeleyev published his conclusions in the first place by sending, early in March 186 9, a leaflet to many Russian and foreign scientists. It gave his system of elements based on their atomic weights and chemical resemblance. On the 18th March that year his paper on the subject was read at the meeting of the Russian Chemical Society, and two months later the Society's Journal published his article entitled "The correlation between properties of elements and their atomic weight". -
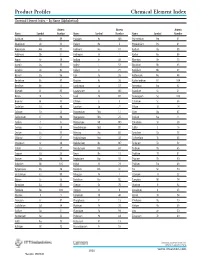
Product Profiles Chemical Element Index
Product Profiles Chemical Element Index Chemical Element Index – By Name (Alphabetical) Atomic Atomic Atomic Name Symbol Number Name Symbol Number Name Symbol Number Actinium Ac 89 Hassium Hs 108 Promethium Pm 61 Aluminum Al 13 Helium He 2 Protactinium Pa 91 Americium Am 95 Holmium Ho 67 Radium Ra 88 Antimony Sb 51 Hydrogen H 1 Radon Rn 86 Argon Ar 18 Indium In 49 Rhenium Re 75 Arsenic As 33 Iodine I 53 Rhodium Rh 45 Astatine At 85 Iridium Ir 77 Rubidium Rb 37 Barium Ba 56 Iron Fe 26 Ruthenium Ru 44 Berkelium Bk 97 Krypton Kr 36 Rutherfordium Rf 104 Beryllium Be 4 Lanthanum La 57 Samarium Sm 62 Bismuth Bi 83 Lawrencium Lr 103 Scandium Sc 21 Boron B 5 Lead Pb 82 Seaborgium Sg 106 Bromine Br 35 Lithium Li 3 Selenium Se 34 Cadmium Cd 48 Lutetium Lu 71 Silicon Si 14 Calcium Ca 20 Magnesium Mg 12 Silver Ag 47 Californium Cf 98 Manganese Mn 25 Sodium Na 11 Carbon C 6 Meitnerium Mt 109 Strontium Sr 38 Cerium Ce 58 Mendelevium Md 101 Sulfur S 16 Cesium Cs 55 Mercury Hg 80 Tantalum Ta 73 Chlorine Cl 17 Molybdenum Mo 42 Technetium Tc 43 Chromium Cr 24 Neilsborium Ns 107 Tellurium Te 52 Cobalt Co 27 Neodymium Nd 60 Terbium Tb 65 Copper Cu 29 Neon Ne 10 Thallium Tl 81 Curium Cm 96 Neptunium Np 93 Thorium Th 90 Dubnium Db 105 Nickel Ni 28 Thulium Tm 69 Dysprosium Dy 66 Niobium Nb 41 Tin Sn 50 Einsteinium Es 99 Nitrogen N 7 Titanium Ti 22 Erbium Er 68 Nobelium No 102 Tungsten W 74 Europium Eu 63 Osmian Os 76 Uranium U 92 Fermium Fm 100 Oxygen O 8 Vanadium V 23 Fluorine F 9 Palladium Pd 46 Xenon Xi 54 Francium Fr 87 Phosphorus P 15 Ytterbium Yb 70 Gadolinium -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants
UNLV Theses, Dissertations, Professional Papers, and Capstones 5-1-2015 Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants John Dustin Despotopulos University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Chemistry Commons Repository Citation Despotopulos, John Dustin, "Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2345. http://dx.doi.org/10.34917/7645877 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. INVESTIGATION OF FLEROVIUM AND ELEMENT 115 HOMOLOGS WITH MACROCYCLIC EXTRACTANTS By John Dustin Despotopulos Bachelor of Science in Chemistry University of Oregon 2010 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy -

Python Module Index 79
mendeleev Documentation Release 0.9.0 Lukasz Mentel Sep 04, 2021 CONTENTS 1 Getting started 3 1.1 Overview.................................................3 1.2 Contributing...............................................3 1.3 Citing...................................................3 1.4 Related projects.............................................4 1.5 Funding..................................................4 2 Installation 5 3 Tutorials 7 3.1 Quick start................................................7 3.2 Bulk data access............................................. 14 3.3 Electronic configuration......................................... 21 3.4 Ions.................................................... 23 3.5 Visualizing custom periodic tables.................................... 25 3.6 Advanced visulization tutorial...................................... 27 3.7 Jupyter notebooks............................................ 30 4 Data 31 4.1 Elements................................................. 31 4.2 Isotopes.................................................. 35 5 Electronegativities 37 5.1 Allen................................................... 37 5.2 Allred and Rochow............................................ 38 5.3 Cottrell and Sutton............................................ 38 5.4 Ghosh................................................... 38 5.5 Gordy................................................... 39 5.6 Li and Xue................................................ 39 5.7 Martynov and Batsanov........................................ -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Ment 98), Einsteinium (Element 99), Fermium (Element 100), Mendelevium (Element Downloaded by Guest on September 26, 2021 472 PHYSICS: G
VOL. 45, 1959 PHYSICS: G. T. SEABORG 471 of californium isotopes. The horizontal broken line labeled "0.02%" indicates the peak height for a group which would have that intensity relative to the total alpha particles. It is seen that the general background is considerably higher than this value, and, in fact, some of the peaks which are sketched in are hardly justifi- able in view of the scatter of the data. The bottom part of Figure 8 shows the same part of the spectrum taken under much improved conditions, as shown by the line illustrating the peak height of 0.02 per cent. All of the peaks are seen much more clearly and, in addition, one or two have showed up which were completely ob- scured under the other conditions. The principal change in technique which re- sulted in this improvement consisted of lining the interior of the spectrograph with very-low-atomic-number material which has the desirable effect of reducing low- angle scattering of alpha particles. In summary, we may say that during the past few years very important ad- vances in theory, as well as in experimental technique, are beginning to result in a systematic assignment of nuclear spectroscopic states and an interpretation of these states in terms of some detailed knowledge of nuclear structure. In this discussion, attention has been confined to one aspect of a very broad problem, namely, that having to do with spectroscopic states in the heavy-element region deduced from the alpha-decay process. There are many other experimental methods which are brought to bear on this problem, some of which have been touched upon in a peripheral sense in this discussion and others not mentioned at all. -

Synthesis of Superheavy Elements by Cold Fusion
Radiochim. Acta 99, 405–428 (2011) / DOI 10.1524/ract.2011.1854 © by Oldenbourg Wissenschaftsverlag, München Synthesis of superheavy elements by cold fusion By S. Hofmann1,2,∗ 1 GSI Helmholtzzentrum für Schwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany 2 Institut für Kernphysik, Goethe-Universität, Max von Laue-Straße 1, 60438 Frankfurt, Germany (Received February 3, 2011; accepted in revised form April 18, 2011) Superheavy elements / Synthesis / Cold fusion / in 1972, and the RILAC (variable-frequency linear acceler- Cross-sections / Decay properties / Recoil separators / ator) at the RIKEN Nishina Center in Saitama near Tokio in Detector systems 1980. In the middle of the 1960s the concept of the macrosco- pic-microscopic model for calculating binding energies Summary. The new elements from Z = 107 to 112 were of nuclei also at large deformations was invented by synthesized in cold fusion reactions based on targets of lead V. M. Strutinsky [3]. Using this method, a number of the and bismuth. The principle physical concepts are presented measured phenomena could be naturally explained by con- which led to the application of this reaction type in search sidering the change of binding energy as function of defor- experiments for new elements. Described are the technical mation. In particular, it became possible now to calculate the developments from early mechanical devices to experiments with recoil separators. An overview is given of present ex- binding energy of a heavy fissioning nucleus at each point of periments which use cold fusion for systematic studies and the fission path and thus to determine the fission barrier. synthesis of new isotopes.