Development of Calcined Clays As Pozzolanic Additions in Portland Cement Concrete Mixtures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manuscript Details
Manuscript Details Manuscript number MATERIALSCHAR_2019_439_R1 Title THE EFFECTS OF MgO, Na2O AND SO3 ON INDUSTRIAL CLINKERING PROCESS: PHASE COMPOSITION, POLYMORPHISM, MICROSTRUCTURE AND HYDRATION, USING A MULTIDISCIPLINARY APPROACH. Article type Research Paper Abstract The present investigation deals with how minor elements (their oxides: MgO, Na2O and SO3) in industrial kiln feeds affect (i) chemical reactions upon clinkering, (ii) resulting phase composition and microstructure of clinker, (iii) hydration process during cement production. Our results show that all these points are remarkably sensitive to the combination and interference effects between the minor chemical species mentioned above. Upon clinkering, all the industrial raw meals here used exhibit the same formation temperature and amount of liquid phase. Minor elements are preferentially hosted by secondary phases, such as periclase. Conversely, the growth rate of the main clinker phases (alite and belite) is significantly affected by the nature and combination of minor oxides. MgO and Na2O give a very fast C3S formation rate at T > 1450 K, whereas Na2O and SO3 boost C2S. After heating, if SO3 occurs in combination with MgO and/or Na2O, it does not inihibit the C3S crystallisation as expected. Rather, it promotes the stabilisation of M1-C3S, thus indirectly influencing the aluminate content, too. MgO increseases the C3S amount and promotes the stabilisation of M3-C3S, when it is in combination with Na2O. Na2O seems to be mainly hosted by calcium aluminate structure, but it does not induce the stabilisation of the orhtorhombic polymorph, as supposed to occur. Such features play a key role in predicting the physical-mechanical performance of a final cement (i.e. -
Cement Data Sheet
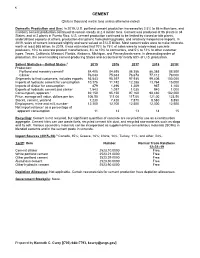
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

Evaluation of Ash Pozzolanic Activity by Means of the Strength Activity Index Test, Frattini Test and DTA/TG Analysis
ISSN 1330-3651 (Print), ISSN 1848-6339 (Online) https://doi.org/10.17559/TV-20171203193229 Original scientific paper Evaluation of Ash Pozzolanic Activity by Means of the Strength Activity Index Test, Frattini Test and DTA/TG Analysis Sabina KRAMAR, Vilma DUCMAN Abstract: The pozzolanic activity of five different types of ash was studied using various direct and indirect methods. In addition to strength activity index (SAI) determination and the Frattini test, ash pozzolanicity was assessed via differential thermal and thermogravimetric analyses (DTA/TG) after curing for 7, 28 and 90 days. The results showed that, due to their respective mineralogical, chemical and physical characteristics, the ashes exhibited different levels of pozzolanic activity in terms of the amount of lime with which they could chemically bind, as well as reaction kinetics. Although SAI and Frattini test results were not in agreement in the case of some of the ashes, DTA/TG analysis revealed that a certain amount of portlandite was consumed, thus confirming the occurrence of a pozzolanic reaction. The results also showed that ashes with higher amounts of reactive SiO2 were more reactive, while those with higher BET surface areas displayed a faster pozzolanic reaction rate. Keywords: biomass ash, DTA/TG, fly ash, Frattini test, pozzolanic activity, SAI 1 INTRODUCTION [7]. The utilisation potential of fly ash is mainly controlled by its chemical composition, but also by particle size, Supplementary cementitious materials (SCMs), amorphous phase content, specific surface area, including fly ash, ground granulated blast furnace slag, morphology and surface texture [8-10]. In contrast to those silica fume, calcined clays and natural pozzolans, are obtained from coal combustion, biomass fly ashes are increasingly used in the construction sector, e.g. -

96 Quality Control of Clinker Products by SEM and XRF Analysis Ziad Abu
ACXRI '96 Quality Control of Clinker Products By SEM and XRF Analysis Ziad Abu Kaddourah and Khairun Azizi MY9700786 School of Materials and Mineral Resources Eng., Universiti Sains Malaysia 31750 Tronoh, Perak, Malaysia. ABSTRACT The microstructure and chemical properties of industrial Portland cement clinkers have been examined by SEM and XRF methods to establish the nature of the clinkers and how variations in the clinker characteristics can be used to control the clinker quality. The clinker nodules were found to show differences in the chemical composition and microstructure between the inner and outer parts of the clinker nodules. Microstructure studies of industrial Portland cement clinker have shown that the outer part of the nodules are enriched in silicate more than the inner part. There is better crystallization and larger alite crystal 9ize in the outer part than in the inner part. The alite crystal size varied between 16.2-46.12um. The clinker chemical composition was found to affect the residual >45um, where a higher belite content causes an increase in the residual >45um in the cement product and will cause a decrease in the concrete strength of the cement product. The aluminate and ferrite crystals and the microcracks within the alite crystal are clear in some clinker only. The quality of the raw material preparation, burning and cooling stages can be controlled using the microstructure of the clinker product. INTRODUCTION Examination of manufactured industrial clinkers using the Scanning Electron Microscope (SEM) is usually conducted to study problems that can't be defined by the normal quality control procedures. Such a study can be used to give better information and knowledge about clinkers characteristics and how variations in the clinker characteristics are affected by variations in the various stages during the manufacturing process. -

Thermodynamics of Portland Cement Clinkering
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aberdeen University Research Archive Thermodynamics of Portland Cement Clinkering Theodore Hanein1, Fredrik P. Glasser2, Marcus Bannerman1* 1. School of Engineering, University of Aberdeen, AB24 3UE, United Kingdom 2. Department of Chemistry, University of Aberdeen, AB24 3UE, United Kingdom Abstract The useful properties of cement arise from the assemblage of solid phases present within the cement clinker. The phase proportions produced from a given feedstock are often predicted using well-established stoichiometric relations, such as the Bogue equations. These approaches are based on a single estimation of the stable phases produced under standard processing conditions and so they are limited in their general application. This work presents a thermodynamic database and simple equilibrium model which is capable of predicting cement phase stability across the full range of kiln temperatures, including the effect of atmospheric conditions. This is termed a “reaction path” and benchmarks the kinetics of processes occurring in the kiln. Phase stability is calculated using stoichiometric phase data and Gibbs free energy minimization under the constraints of an elemental balance. Predictions of stable phases and standard phase diagrams are reproduced for the manufacture of ordinary Portland cement and validated against results in the literature. The stability of low-temperature phases, which may be important in kiln operation, is explored. Finally, an outlook on future applications of the database in optimizing cement plant operation and development of new cement formulations is provided. Originality This report details corrections to reference thermodynamic data which are relevant for cement thermodynamic calculations. -

Portland Cement Clinker
Conforms to HazCom 2012/United States Safety Data Sheet Portland Cement Clinker Section 1. Identification GHS product identifier: Portland Cement Clinker Chemical name: Calcium compounds, calcium silicate compounds, and other calcium compounds containing iron and aluminum make up the majority of this product. Other means of identification: Clinker, Cement Clinker Relevant identified uses of the substance or mixture and uses advised against: Raw material for cement manufacturing. Supplier’s details: 300 E. John Carpenter Freeway, Suite 1645 Irving, TX 75062 (972) 653-5500 Emergency telephone number (24 hours): CHEMTREC: (800) 424-9300 Section 2. Hazards Identification Overexposure to portland cement clinker can cause serious, potentially irreversible skin or eye damage in the form of chemical (caustic) burns, including third degree burns. The same serious injury can occur if wet or moist skin has prolonged contact exposure to dry portland cement clinker. OSHA/HCS status: This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the SKIN CORROSION/IRRITATION – Category 1 substance or mixture: SERIOUS EYE DAMAGE/EYE IRRITATION – Category 1 SKIN SENSITIZATION – Category 1 CARCINOGENICITY/INHALATION – Category 1A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) [Respiratory tract irritation] – Category 3 GHS label elements Hazard pictograms: Signal word: Danger Hazard statements: Causes severe skin burns and eye damage. May cause an allergic skin reaction. May cause respiratory irritation. May cause cancer. Precautionary statements: Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Avoid breathing dust. Use outdoors in a well ventilated area. Wash any exposed body parts thouroughly after handling. -

A Study of Strength Activity Index of Ground Coarse Fly Ash with Portland Cement
R ESEARCH ARTICLE ScienceAsia 25 (1999) : 223-229 A Study of Strength Activity Index of Ground Coarse Fly Ash with Portland Cement Chai Jaturapitakkul*, Kraiwood Kiattikomol and Smith Songpiriyakij Department of Civil Engineering, King Mongkut's University of Technology Thonburi, Bangkok 10140, Thailand. * Corresponding author. Received 16 February 1999 ABSTRACT Fly ash, from Mae Moh power plant, was classified by air classifier to yield fine and coarse fractions. The coarse fly ash was ground and classified again into 3 sizes. Four different sizes of fly ash from the process including the original fly ash were replaced cement 20% by weight to make mortars. Chemical and physical properties of all fly ashes were tested. Setting times of fly ash-cement pastes and compressive strength of fly ash-cement mortars were investigated, and compared with those of the control. The results revealed that slight change in chemical composition of processed fly ashes did not give much effect on the compressive strength, whereas, the fineness of fly ash played a more important factor on the compressive strength development rate. More than 110% of strength activity index of ground coarse fly ash mortar can be achieved as early as 1 to 3 days since the coarse fly ash is not in crystalline phase. KEYWORDS: fly ash, coarse fly ash, fineness, mortar, strength activity index. INTRODUCTION showed that the small particle size of fly ash was a good source of pozzolanic material7. Later, classified Fly ash, as defined by ACI 116,1 is the finely fly ash by air cycling was developed at King divided residue resulting from the combustion of Mongkut’s Institute of Technology Thonburi ground or powdered coal and which is transported (KMITT) in 1996 which be able to select the mean from the firebox through the boiler by flue gases; particle size down to 3 micron.8 The test results of known in UK as pulverized-fuel ash. -

Delayed Ettringite Formation
Ettringite Formation and the Performance of Concrete In the mid-1990’s, several cases of premature deterioration of concrete pavements and precast members gained notoriety because of uncertainty over the cause of their distress. Because of the unexplained and complex nature of several of these cases, considerable debate and controversy have arisen in the research and consulting community. To a great extent, this has led to a misperception that the problems are more prevalent than actual case studies would indicate. However, irrespective of the fact that cases of premature deterioration are limited, it is essential to address those that have occurred and provide practical, technically sound solutions so that users can confidently specify concrete in their structures. Central to the debate has been the effect of a compound known as ettringite. The objectives of this paper are: Fig. 1. Portland cements are manufactured by a process that combines sources of lime (such as limestone), silica and • to define ettringite and its form and presence in concrete, alumina (such as clay), and iron oxide (such as iron ore). Appropriately proportioned mixtures of these raw materials • to respond to questions about the observed problems and the are finely ground and then heated in a rotary kiln at high various deterioration mechanisms that have been proposed, and temperatures, about 1450 °C (2640 °F), to form cement compounds. The product of this process is called clinker • to provide some recommendations on designing for durable (nodules at right in above photo). After cooling, the clinker is concrete. interground with about 5% of one or more of the forms of Because many of the questions raised relate to cement character- calcium sulfate (gypsum shown at left in photo) to form portland cement. -

Cementing Qualities of the Calcium Aluminates
DEPARTMENT OF COMMERCE Technologic Papers of THE Bureau of Standards S. W. STRATTON, Director No. 19 7 CEMENTING QUALITIES OF THE CALCIUM ALUMINATES BY P. H. BATES, Chemist Bureau of Standards SEPTEMBER 27, 1921 PRICE, 10 CENTS Sold only by the Superintendent of Documents, Government Printing Office Washington, D. C. WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CEMENTING QUALITIES OF THE CALCIUM ALBUMI- NATES Bv P. H. Bates ABSTRACT four calcium aluininates (3CaO.Al 5Ca0.3Al , CaO.Al The 2 3 , 2 3 2 3 , 3Ca0.5Al 2 3 ) have been made in a pure condition and their cementing qualities determined. The first two reacted so energetically with water that too rapid set resulted to make them usable commercially. The last two set more slowly and developed very high strengths at early periods. These two alurninates high in alumina were later made in a pure and impure condition in larger quantities in a rotary kiln and concrete was made from the resulting ground clinker. A 1:1.5:5.5 gravel concrete developed in 24 hours as high strength as a similarly proportioned Portland cement concrete would have developed in 28 days. Recent investigations * have shown that lime can combine with alumina only in the following molecular proportions: 3CaO. A1 5Ca0.3Al , CaO.Al 3Ca0.5Al . These investiga- 2 3 , 2 3 2 3 , 2 3 tions also show that the tricalcium aluminate is the only aluminate present in Portland cement of normal composition and normal properties. Later these results were confirmed by work carried on at this Bureau and published as Technologic Paper No. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

The Effect of Various Chemical Activators on Pozzolanic Reactivity: a Review
Scientific Research and Essays Vol. 7(7), pp. 719-729, 23 February, 2012 Available online at http://www.academicjournals.org/SRE DOI: 10.5897/SRE10.858 ISSN 1992-2248 ©2012 Academic Journals Review The effect of various chemical activators on pozzolanic reactivity: A review A. Abdullah1,2, M. S Jaafar3*, Y. H Taufiq-Yap1, A. Alhozaimy4 and A. Al-Negheimish4 and J. Noorzaei3,5 1Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. 2Faculty of Applied Sciences, Universiti Teknologi Mara, 72000 Kuala Pilah, Negeri Sembilan, Malaysia. 3Department of Civil Engineering, Faculty of Engineering, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. 4Centre of Excellent for Concrete Research and Testing (CoE-CRT), Faculty of Engineering, King Saud University, Riyadh, Saudi Arabia. 5Institute of Advance Technology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. Accepted 26 January, 2011 Pozzolans are being extensively used as a partial cement replacement in the production of Portland cement concrete. The advantages of using pozzolans are lower costs and better durability, however it requires longer setting time and the strength development at the initial age is slower. Different methods have been developed to overcome these disadvantages and to enhance the reactivity of natural pozzolans. There are three ways which have been used to improve the pozzolanic reactivity; it includes mechanical method, thermal method and chemical method. The utilization of the chemical method as an activator of pozzolanic reactivity has received considerable attention in recent years. In this paper the effect of using different chemical activators on pozzolanic activity of a pozzolan is summarized by reviewing previously published work. -

Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China
minerals Article Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China Lei Lu 1,2, Yan Liu 3,4,*, Huichuan Liu 5,*, Zhi Zhao 2, Chenghui Wang 2 and Xiaochun Xu 1 1 School of Resources and Environmental Engineering, Hefei University of Technology, Hefei 230009, China; [email protected] (L.L.); [email protected] (X.X.) 2 MLR Key Laboratory of Metallogeny and Mineral Resource Assessment, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing 100037, China; [email protected] (Z.Z.); [email protected] (C.W.) 3 Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou 511458, China 4 Key Laboratory of Deep-Earth Dynamics of Ministry of Natural Resources, Institute of Geology, Chinese Academy of Geological Science, Beijing 100037, China 5 State Key Laboratory of Petroleum Resources and Prospecting, China University of Petroleum (Beijing), Beijing 102249, China * Correspondence: [email protected] (Y.L.); [email protected] (H.L.) Received: 3 November 2020; Accepted: 2 December 2020; Published: 12 December 2020 Abstract: Granites are assumed to be the main source of heavy rare-earth elements (HREEs), which have important applications in modern society. However, the geochemical and petrographic characteristics of such granites need to be further constrained, especially as most granitic HREE deposits have undergone heavy weathering. The LC batholith comprises both fresh granite and ion-adsorption-type HREE deposits, and contains four main iRee (ion-adsorption-type REE) deposits: the Quannei (QN), Shangyun (SY), Mengwang (MW), and Menghai (MH) deposits, which provide an opportunity to elucidate these characteristics The four deposits exhibit light REE (LREE) enrichment, and the QN deposit is also enriched in HREEs.