Scattergood Seawater Desalination Pilot Plant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carol Raskin
Carol Raskin Artistic Images Make-Up and Hair Artists, Inc. Miami – Office: (305) 216-4985 Miami - Cell: (305) 216-4985 Los Angeles - Office: (310) 597-1801 FILMS DATE FILM DIRECTOR PRODUCTION ROLE 2019 Fear of Rain Castille Landon Pinstripe Productions Department Head Hair (Kathrine Heigl, Madison Iseman, Israel Broussard, Harry Connick Jr.) 2018 Critical Thinking John Leguizamo Critical Thinking LLC Department Head Hair (John Leguizamo, Michael Williams, Corwin Tuggles, Zora Casebere, Ramses Jimenez, William Hochman) The Last Thing He Wanted Dee Rees Elena McMahon Productions Additional Hair (Miami) (Anne Hathaway, Ben Affleck, Willem Dafoe, Toby Jones, Rosie Perez) Waves Trey Edward Shults Ultralight Beam LLC Key Hair (Sterling K. Brown, Kevin Harrison, Jr., Alexa Demie, Renee Goldsberry) The One and Only Ivan Thea Sharrock Big Time Mall Productions/Headliner Additional Hair (Bryan Cranston, Ariana Greenblatt, Ramon Rodriguez) (U. S. unit) 2017 The Florida Project Sean Baker The Florida Project, Inc. Department Head Hair (Willem Dafoe, Bria Vinaite, Mela Murder, Brooklynn Prince) Untitled Detroit City Yann Demange Detroit City Productions, LLC Additional Hair (Miami) (Richie Merritt Jr., Matthew McConaughey, Taylour Paige, Eddie Marsan, Alan Bomar Jones) 2016 Baywatch Seth Gordon Paramount Worldwide Additional Hair (Florida) (Dwayne Johnson, Zac Efron, Alexandra Daddario, David Hasselhoff) Production, Inc. 2015 The Infiltrator Brad Furman Good Films Production Department Head Hair (Bryan Cranston, John Leguizamo, Benjamin Bratt, Olympia -

Air Line Pilots Page 5 Association, International Our Skies
March 2015 ALSO IN THIS ISSUE: » Landing Your » Known Crewmember » Sleep Apnea Air Dream Job page 20 page 29 Update page 28 Line PilOt Safeguarding Official Journal of the Air Line Pilots page 5 Association, International Our Skies Follow us on Twitter PRINTED IN THE U.S.A. @wearealpa Sponsored Airline- Career Track ATP offers the airline pilot career training solution with a career track from zero time to 1500 hours sponsored by ATP’s airline alliances. Airline Career month FAST TRACK Demand for airline pilots and ATP graduates is soaring, Pilot Program with the “1500 hour rule” and retirements at the majors. AIRLINES Airlines have selected ATP as a preferred training provider to build their pilot pipelines Private, Instrument, Commercial Multi Also available with... & Certified Flight Instructor (Single, Multi 100 Hours Multi-Engine Experience with the best training in the fastest & Instrument) time frame possible. 225 Hours Flight Time / 100 Multi 230 Hours Flight Time / 40 Multi In the Airline Career Pilot Program, your airline Gain Access to More Corporate, Guaranteed Flight Instructor Job Charter, & Multi-Engine Instructor interview takes place during the commercial phase Job Opportunities of training. Successful applicants will receive a Airline conditional offer of employment at commercial phase of training, based on building Fly Farther & Faster with Multi- conditional offer of employment from one or more of flight experience to 1500 hours in your guaranteed Engine Crew Cross-Country ATP’s airline alliances, plus a guaranteed instructor CFI job. See website for participating airlines, Experience job with ATP or a designated flight school to build admissions, eligibility, and performance requirements. -

Infant and Toddler Contracted Slots Pilot Program: Evaluation Report Chad Dorn, Phd
Infant and Toddler Contracted Slots Pilot Program: Evaluation Report Chad Dorn, PhD. August 2020 2 Propulscreating force leading toon movement Contents ACKNOWLEDGEMENT............................................................................................................... 4 OVERVIEW OF INFANT TODDLER CONTRACTED SLOTS PILOT ....................................... 5 THE EVALUATION........................................................................................................................ 7 Implementation Science (IS) Framework............................................................................... 7 Building the capacity to effectively implement...................................................................... 9 Implementation Teams............................................................................................... 9 Data-Informed Feedback Loops................................................................................. 9 Implementation Infrastructure.................................................................................. 10 Data Collection and Timeline................................................................................. ............... 10 Data Sources and Collection .................................................................................................. 11 Administrative Data.................................................................................................... 11 Program Director Survey – Pre and Post................................................................... -

Tracing Fairy Tales in Popular Culture Through the Depiction of Maternity in Three “Snow White” Variants
University of Louisville ThinkIR: The University of Louisville's Institutional Repository College of Arts & Sciences Senior Honors Theses College of Arts & Sciences 5-2014 Reflective tales : tracing fairy tales in popular culture through the depiction of maternity in three “Snow White” variants. Alexandra O'Keefe University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/honors Part of the Children's and Young Adult Literature Commons, and the Comparative Literature Commons Recommended Citation O'Keefe, Alexandra, "Reflective tales : tracing fairy tales in popular culture through the depiction of maternity in three “Snow White” variants." (2014). College of Arts & Sciences Senior Honors Theses. Paper 62. http://doi.org/10.18297/honors/62 This Senior Honors Thesis is brought to you for free and open access by the College of Arts & Sciences at ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in College of Arts & Sciences Senior Honors Theses by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. O’Keefe 1 Reflective Tales: Tracing Fairy Tales in Popular Culture through the Depiction of Maternity in Three “Snow White” Variants By Alexandra O’Keefe Submitted in partial fulfillment of the requirements for Graduation summa cum laude University of Louisville March, 2014 O’Keefe 2 The ability to adapt to the culture they occupy as well as the two-dimensionality of literary fairy tales allows them to relate to readers on a more meaningful level. -

16. Watershed Assets Assessment Report
16. Watershed Assets Assessment Report Jingfen Sheng John P. Wilson Acknowledgements: Financial support for this work was provided by the San Gabriel and Lower Los Angeles Rivers and Mountains Conservancy and the County of Los Angeles, as part of the “Green Visions Plan for 21st Century Southern California” Project. The authors thank Jennifer Wolch for her comments and edits on this report. The authors would also like to thank Frank Simpson for his input on this report. Prepared for: San Gabriel and Lower Los Angeles Rivers and Mountains Conservancy 900 South Fremont Avenue, Alhambra, California 91802-1460 Photography: Cover, left to right: Arroyo Simi within the city of Moorpark (Jaime Sayre/Jingfen Sheng); eastern Calleguas Creek Watershed tributaries, classifi ed by Strahler stream order (Jingfen Sheng); Morris Dam (Jaime Sayre/Jingfen Sheng). All in-text photos are credited to Jaime Sayre/ Jingfen Sheng, with the exceptions of Photo 4.6 (http://www.you-are- here.com/location/la_river.html) and Photo 4.7 (digital-library.csun.edu/ cdm4/browse.php?...). Preferred Citation: Sheng, J. and Wilson, J.P. 2008. The Green Visions Plan for 21st Century Southern California. 16. Watershed Assets Assessment Report. University of Southern California GIS Research Laboratory and Center for Sustainable Cities, Los Angeles, California. This report was printed on recycled paper. The mission of the Green Visions Plan for 21st Century Southern California is to offer a guide to habitat conservation, watershed health and recreational open space for the Los Angeles metropolitan region. The Plan will also provide decision support tools to nurture a living green matrix for southern California. -

The Relative Cost-Effectiveness of Retaining Versus Accessing Air Force Pilots
The Relative Cost-Effectiveness of Retaining Versus Accessing Air Force Pilots Michael G. Mattock, Beth J. Asch, James Hosek, Michael Boito C O R P O R A T I O N For more information on this publication, visit www.rand.org/t/RR2415 Library of Congress Cataloging-in-Publication Data is available for this publication. ISBN: 978-1-9774-0204-2 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2019 RAND Corporation R® is a registered trademark. Cover: Getty Images/Fanatic Studio. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions. The RAND Corporation is a research organization that develops solutions to public policy challenges to help make communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND Make a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Preface The research discussed in this report was conducted for a project to develop an analytic capability for determining the efficient amount of special and incentive (S&I) pays for rated officers in the U.S. -

OJ Episode 1, FINAL, 6-3-15.Fdx Script
EPISODE 1: “FROM THE ASHES OF TRAGEDY” Written by Scott Alexander and Larry Karaszewski Based on “THE RUN OF HIS LIFE” By Jeffrey Toobin Directed by Ryan Murphy Fox 21 FX Productions Color Force FINAL 6-3-15 Ryan Murphy Television ALL RIGHTS RESERVED. COPYRIGHT © 2015 TWENTIETH CENTURY FOX FILM CORPORATION. NO PORTION OF THIS SCRIPT MAY BE PERFORMED, PUBLISHED, REPRODUCED, SOLD, OR DISTRIBUTED BY ANY MEANS OR QUOTED OR PUBLISHED IN ANY MEDIUM, INCLUDING ANY WEB SITE, WITHOUT PRIOR WRITTEN CONSENT OF TWENTIETH CENTURY FOX FILM CORPORATION. DISPOSAL OF THIS SCRIPT COPY DOES NOT ALTER ANY OF THE RESTRICTIONS SET FORTH ABOVE. 1 ARCHIVE FOOTAGE - THE RODNEY KING BEATING. Grainy, late-night 1 video. An AFRICAN-AMERICAN MAN lies on the ground. A handful of white LAPD COPS stand around, watching, while two of them ruthlessly BEAT and ATTACK him. ARCHIVE FOOTAGE - THE L.A. RIOTS. The volatile eruption of a city. Furious AFRICAN-AMERICANS tear Los Angeles apart. Trash cans get hurled through windows. Buildings burn. Cars get overturned. People run through the streets. Faces are angry, frustrated, screaming. The NOISE and FURY and IMAGES build, until -- SILENCE. Then, a single CARD: "TWO YEARS LATER" CUT TO: 2 EXT. ROCKINGHAM HOUSE - LATE NIGHT 2 ANGLE on a BRONZE STATUE of OJ SIMPSON, heroic in football regalia. Larger than life, inspiring. It watches over OJ'S estate, an impressive Tudor mansion on a huge corner lot. It's June 12, 1994. Out front, a young LIMO DRIVER waits. He nervously checks his watch. Then, OJ SIMPSON comes rushing from the house. -

Chapter 3-Deicing /Anti-Icing Fluids
When in Doubt... Small and Large Aircraft Aircraft Critical Surface Contamination Training For Aircrew and Groundcrew Seventh Edition December 2004 2 When in Doubt…TP 10643E How to Use This Manual This manual has been organized and written in chapter and summary format. Each chapter deals with certain topics that are reviewed in a summary at the end of the chapter. The manual is divided into two parts: Part 1-for aircrew and groundcrew; Part 2-additional information for ground crew. The final chapter contains questions that any operator may utilize for their ground icing program examination. The references for each question are listed to assist with answers. The Holdover Tables (HOT) included in the appendix are solely for the use with the examination questions and must not be used for operations. Contact Transport Canada from the addresses located later in this document for the latest HOT. Warnings These are used throughout this manual and are items, which will result in: damage to equipment, personal injury and/or loss of life if not carefully followed. Cautions These are used throughout this manual and are items, which may result in: damage to equipment, personal injury or loss of life if not carefully followed. Notes These are items that are intended to further explain details and clarify by amplifying important information. Should Implies that it is advisable to follow the suggested activity, process or practice. Must Implies that the suggested activity, process or practice needs to be followed because there are significant safety -

Chapter: 2. En Route Operations
Chapter 2 En Route Operations Introduction The en route phase of flight is defined as that segment of flight from the termination point of a departure procedure to the origination point of an arrival procedure. The procedures employed in the en route phase of flight are governed by a set of specific flight standards established by 14 CFR [Figure 2-1], FAA Order 8260.3, and related publications. These standards establish courses to be flown, obstacle clearance criteria, minimum altitudes, navigation performance, and communications requirements. 2-1 fly along the centerline when on a Federal airway or, on routes other than Federal airways, along the direct course between NAVAIDs or fixes defining the route. The regulation allows maneuvering to pass well clear of other air traffic or, if in visual meteorogical conditions (VMC), to clear the flightpath both before and during climb or descent. Airways Airway routing occurs along pre-defined pathways called airways. [Figure 2-2] Airways can be thought of as three- dimensional highways for aircraft. In most land areas of the world, aircraft are required to fly airways between the departure and destination airports. The rules governing airway routing, Standard Instrument Departures (SID) and Standard Terminal Arrival (STAR), are published flight procedures that cover altitude, airspeed, and requirements for entering and leaving the airway. Most airways are eight nautical miles (14 kilometers) wide, and the airway Figure 2-1. Code of Federal Regulations, Title 14 Aeronautics and Space. flight levels keep aircraft separated by at least 500 vertical En Route Navigation feet from aircraft on the flight level above and below when operating under VFR. -

Feminism and Magic in Snow White
Dittmeier 1 Feminism and Magic in Snow White Magic is something that has always been very prevalent in fairy tales. Many fairy tales include young women—complacent, innocent, and invariably beautiful young women—who are either terrorized by an evil witch, helped out of a bad circumstance by a fairy godmother, or both. They are expected to handle their misfortune gracefully and show kindness even to their enemies, which they are often rewarded for, and generally this reward is marriage. However, some of these fairy tales can be seen through a more feminist lens. Magical fairy tale women do not have to just be either evil or the bumbling fairy godmother who helps the heroine get her marriage reward. Snow White, for example, has more control over her story than may be apparent. There is a hidden feminist ideology in Snow White that we can see through Snow White’s use of magic. Witches—women who can use magic—are, in modern times, a feminist symbol. The witch is a woman who has her own power, and in the case of some stories, Macbeth, for example, they have power to drive the plot (Guadagnino, 2018). The witch can be a positive symbol for a woman who has her own agency. If we look closely, we can see clues that indicate that Snow White is not only a witch, but that she is using her own power to drive her story along. Magic is evident from the very beginning of Snow White. Snow White’s mother is sewing by the open window, and she pricks her finger. -

The Disappearance Of
georgeTHE DISAPPEARANCE OF SNOW In 1980, a Boca Raton businessman piloted a news helicopter to a remote island in the Bahamas to cover a story that ultimately helped to topple a government. What happened to him remains a mystery that haunts his family to this day. By GASPAR GONZÁLEZ he drift chart that Tim and Jeff Snow keep cover a story involving shipwrecked Haitian migrants. shows the ocean currents around the Bahama Snow and his crew got their story—and then became islands, how they might carry a piece of flotsam part of it, when their helicopter, on its return trip to from just off the coast of Williams Island a couple Miami, disappeared somewhere over the water, never to of hundred miles north, to Grand Bahama. To the be found. casual observer, it’s interesting enough to look at, a What happened to Snow and his three passengers reminder of the wondrous workings of nature. is the subject of enduring speculation. Was it an For the brothers, however, the chart represents accident, or, as some believe, the work of drug dealers? something entirely different—a riddle with no Did the Bahamian government, angry at what the answer. What happened to their father that day newsmen had uncovered, have something to do with the he took off from Andros Island in his helicopter? disappearance? His sons say they’d give anything to know It’s been more than 30 years since George Snow, a the truth. prominent Boca Raton businessman-turned-chopper- But the truth, like the currents, can be hard to read pilot, transported an NBC news crew to the Bahamas to sometimes. -
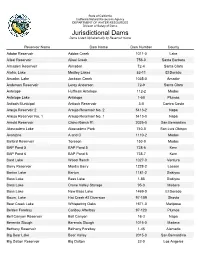
Jurisdictional Dams Listed Alphabetically by Reservoir Name
State of California California Natural Resources Agency DEPARTMENT OF WATER RESOURCES Division of Safety of Dams Jurisdictional Dams Dams Listed Alphabetically by Reservoir Name Reservoir Name Dam Name Dam Number County Adobe Reservoir Adobe Creek 1011-0 Lake Alisal Reservoir Alisal Creek 756-0 Santa Barbara Almaden Reservoir Almaden 72-4 Santa Clara Aloha, Lake Medley Lakes 53-11 El Dorado Amador, Lake Jackson Creek 1035-0 Amador Anderson Reservoir Leroy Anderson 72-9 Santa Clara Antelope Huffman Antelope 112-2 Modoc Antelope Lake Antelope 1-50 Plumas Antioch Municipal Antioch Reservoir 3-0 Contra Costa Araujo Reservoir 2 Araujo Reservoir No. 2 5413-2 Napa Araujo Reservoir No. 1 Araujo Reservoir No. 1 5413-0 Napa Arnold Reservoir Chino Ranch #1 2025-0 San Bernardino Atascadero Lake Atascadero Park 740-0 San Luis Obispo Avanzino A and C 1110-2 Modoc Ballard Reservoir Toreson 153-0 Modoc BAP Pond 5 BAP Pond 5 738-6 Kern BAP Pond 6 BAP Pond 6 738-7 Kern Bard Lake Wood Ranch 1027-0 Ventura Barry Reservoir Mardis Barry 1228-2 Lassen Barton Lake Barton 1181-2 Siskiyou Bass Lake Bass Lake 1-85 Siskiyou Bass Lake Crane Valley Storage 95-3 Madera Bass Lake New Bass Lake 1469-0 El Dorado Baum, Lake Hat Creek #2 Diversion 97-109 Shasta Bear Creek Lake Whispering Oaks 1671-0 Mariposa Belden Forebay Caribou Afterbay 97-120 Plumas Bell Canyon Reservoir Bell Canyon 16-3 Napa Berenda Slough Berenda Slough 1015-0 Madera Bethany Reservoir Bethany Forebay 1-45 Alameda Big Bear Lake Bear Valley 2015-0 San Bernardino Big Dalton Reservoir Big Dalton 32-0 Los Angeles Jurisdictional Dams Dams Listed Alphabetically by Reservoir Name Page 2 of 12 Reservoir Name Dam Name Dam Number County Big Pine Lake Big Pine Creek 6-11 Inyo Big Reservoir Morning Star 325-0 Placer Big Sage Reservoir Big Sage 55-0 Modoc Big Tujunga Reservoir Big Tujunga No.