Gait Transitions in a Phase Oscillator Model of an Insect Central Pattern Generator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interactions Among Diameter, Myelination, and the Na/K Pump Affect Axonal Resilience to High-Frequency Spiking
Interactions among diameter, myelination, and the Na/K pump affect axonal resilience to high-frequency spiking Yunliang Zanga,b and Eve Mardera,b,1 aVolen National Center for Complex Systems, Brandeis University, Waltham, MA 02454; and bDepartment of Biology, Brandeis University, Waltham, MA 02454 Contributed by Eve Marder, June 22, 2021 (sent for review March 25, 2021; reviewed by Carmen C. Canavier and Steven A. Prescott) Axons reliably conduct action potentials between neurons and/or (16–21). It remains unknown how these directly correlated pro- other targets. Axons have widely variable diameters and can be cesses, fast spiking and slow Na+ removal, interact in the control myelinated or unmyelinated. Although the effect of these factors of reliable spike generation and propagation. Furthermore, it is on propagation speed is well studied, how they constrain axonal unclear whether reducing Na+-andK+-current overlap can con- resilience to high-frequency spiking is incompletely understood. sistently decrease the rate of Na+ influx and accordingly enhance Maximal firing frequencies range from ∼1Hzto>300 Hz across axonal reliability to propagate high-frequency spikes. Conse- neurons, but the process by which Na/K pumps counteract Na+ quently, by modeling the process of Na+ removal in unmyelinated influx is slow, and the extent to which slow Na+ removal is com- and myelinated axons, we systematically explored the effects of patible with high-frequency spiking is unclear. Modeling the pro- diameter, myelination, and Na/K pump density on spike propagation cess of Na+ removal shows that large-diameter axons are more reliability. resilient to high-frequency spikes than are small-diameter axons, because of their slow Na+ accumulation. -

Energy Expenditure Computation of a Single Bursting Neuron
Cognitive Neurodynamics (2019) 13:75–87 https://doi.org/10.1007/s11571-018-9503-3 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL ARTICLE Energy expenditure computation of a single bursting neuron 2 1,2 2 2 Fengyun Zhu • Rubin Wang • Xiaochuan Pan • Zhenyu Zhu Received: 17 October 2017 / Revised: 22 August 2018 / Accepted: 28 August 2018 / Published online: 3 September 2018 Ó The Author(s) 2018 Abstract Brief bursts of high-frequency spikes are a common firing pattern of neurons. The cellular mechanisms of bursting and its biological significance remain a matter of debate. Focusing on the energy aspect, this paper proposes a neural energy calculation method based on the Chay model of bursting. The flow of ions across the membrane of the bursting neuron with or without current stimulation and its power which contributes to the change of the transmembrane electrical potential energy are analyzed here in detail. We find that during the depolarization of spikes in bursting this power becomes negative, which was also discovered in previous research with another energy model. We also find that the neuron’s energy consumption during bursting is minimal. Especially in the spontaneous state without stimulation, the total energy con- sumption (2.152 9 10-7 J) during 30 s of bursting is very similar to the biological energy consumption (2.468 9 10-7 J) during the generation of a single action potential, as shown in Wang et al. (Neural Plast 2017, 2017a). Our results suggest that this property of low energy consumption could simply be the consequence of the biophysics of generating bursts, which is consistent with the principle of energy minimization. -

Analyzing Pterosaur Ontogeny and Sexual Dimorphism with Multivariate Allometry Erick Charles Anderson [email protected]
Marshall University Marshall Digital Scholar Theses, Dissertations and Capstones 2016 Analyzing Pterosaur Ontogeny and Sexual Dimorphism with Multivariate Allometry Erick Charles Anderson [email protected] Follow this and additional works at: http://mds.marshall.edu/etd Part of the Animal Sciences Commons, Ecology and Evolutionary Biology Commons, and the Paleontology Commons Recommended Citation Anderson, Erick Charles, "Analyzing Pterosaur Ontogeny and Sexual Dimorphism with Multivariate Allometry" (2016). Theses, Dissertations and Capstones. 1031. http://mds.marshall.edu/etd/1031 This Thesis is brought to you for free and open access by Marshall Digital Scholar. It has been accepted for inclusion in Theses, Dissertations and Capstones by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. ANALYZING PTEROSAUR ONTOGENY AND SEXUAL DIMORPHISM WITH MULTIVARIATE ALLOMETRY A thesis submitted to the Graduate College of Marshall University In partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences by Erick Charles Anderson Approved by Dr. Frank R. O’Keefe, Committee Chairperson Dr. Suzanne Strait Dr. Andy Grass Marshall University May 2016 i ii ii Erick Charles Anderson ALL RIGHTS RESERVED iii Acknowledgments I would like to thank Dr. F. Robin O’Keefe for his guidance and advice during my three years at Marshall University. His past research and experience with reptile evolution made this research possible. I would also like to thank Dr. Andy Grass for his advice during the course of the research. I would like to thank my fellow graduate students Donald Morgan and Tiffany Aeling for their support, encouragement, and advice in the lab and bar during our two years working together. -

Characteristics of Hippocampal Primed Burst Potentiation in Vitro and in the Awake Rat
The Journal of Neuroscience, November 1988, 8(11): 4079-4088 Characteristics of Hippocampal Primed Burst Potentiation in vitro and in the Awake Rat D. M. Diamond, T. V. Dunwiddie, and G. M. Rose Medical Research Service, Veterans Administration Medical Center, and Department of Pharmacology, University of Colorado Health Sciences Center, Denver, Colorado 80220 A pattern of electrical stimulation based on 2 prominent (McNaughton, 1983; Lynch and Baudry, 1984; Farley and Al- physiological features of the hippocampus, complex spike kon, 1985; Byrne, 1987). Of these, a phenomenontermed long- discharge and theta rhythm, was used to induce lasting in- term potentiation (LTP) has received extensive attention be- creases in responses recorded in area CA1 of hippocampal causeit sharesa number of characteristicswith memory. For slices maintained in vitro and from the hippocampus of be- example, LTP has a rapid onset and long duration and is having rats. This effect, termed primed burst (PB) potentia- strengthenedby repetition (Barnes, 1979; Teyler and Discenna, tion, was elicited by as few as 3 stimuli delivered to the 1987). In addition, the decay of LTP is correlated with the time commissural/associational afferents to CAl. The patterns courseof behaviorally assessedforgetting (Barnes, 1979; Barnes of stimulus presentation consisted of a single priming pulse and McNaughton, 1985). The similarity of LTP and memory followed either 140 or 170 msec later by a high-frequency has fostered an extensive characterization of the conditions un- burst of 2-10 pulses; control stimulation composed of un- derlying the initiation of LTP, as well as its biochemical sub- primed high-frequency trains of up to 10 pulses had no en- strates (Lynch and Baudry, 1984; Wigstrijm and Gustafsson, during effect. -

The Logic Behind Neural Control of Breathing Pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C
www.nature.com/scientificreports OPEN The logic behind neural control of breathing pattern Alona Ben-Tal 1, Yunjiao Wang2 & Maria C. A. Leite3 The respiratory rhythm generator is spectacular in its ability to support a wide range of activities and Received: 10 January 2019 adapt to changing environmental conditions, yet its operating mechanisms remain elusive. We show Accepted: 29 May 2019 how selective control of inspiration and expiration times can be achieved in a new representation of Published: xx xx xxxx the neural system (called a Boolean network). The new framework enables us to predict the behavior of neural networks based on properties of neurons, not their values. Hence, it reveals the logic behind the neural mechanisms that control the breathing pattern. Our network mimics many features seen in the respiratory network such as the transition from a 3-phase to 2-phase to 1-phase rhythm, providing novel insights and new testable predictions. Te mechanism for generating and controlling the breathing pattern by the respiratory neural circuit has been debated for some time1–6. In 1991, an area of the brainstem, the pre Bötzinger Complex (preBötC), was found essential for breathing7. An isolated single PreBötC neuron could generate tonic spiking (a non-interrupted sequence of action potentials), bursting (a repeating pattern that consists of a sequence of action potentials fol- lowed by a time interval with no action potentials) or silence (no action potentials)8. Tese signals are transmit- ted, through other populations of neurons, to spinal motor neurons that activate the respiratory muscle4,6. Te respiratory muscle contracts when it receives a sequence of action potentials from the motor neurons and relaxes when no action potentials arrive9. -

Running Birds from Quail to Ostrich Prioritise Leg Safety and Economy
© 2014. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2014) 217, 3786-3796 doi:10.1242/jeb.102640 RESEARCH ARTICLE Don’t break a leg: running birds from quail to ostrich prioritise leg safety and economy on uneven terrain Aleksandra V. Birn-Jeffery1,*,‡, Christian M. Hubicki2,‡, Yvonne Blum1, Daniel Renjewski2, Jonathan W. Hurst2 and Monica A. Daley1,§ ABSTRACT Daley, 2012; Dial, 2003; Jindrich et al., 2007; Rubenson et al., Cursorial ground birds are paragons of bipedal running that span a 2004). These athletes span the broadest body mass range among 500-fold mass range from quail to ostrich. Here we investigate the extant bipeds, over 500-fold from quail to ostrich. Birds thus provide task-level control priorities of cursorial birds by analysing how they a natural animal model for understanding the functional demands of negotiate single-step obstacles that create a conflict between body striding bipedalism and how these demands change with body size stability (attenuating deviations in body motion) and consistent leg (Gatesy and Biewener, 1991; Hutchinson and Garcia, 2002; Roberts force–length dynamics (for economy and leg safety). We also test the et al., 1998a). hypothesis that control priorities shift between body stability and leg Here, we ask two questions fundamental to locomotor behaviour: safety with increasing body size, reflecting use of active control to (1) what are the task-level leg control priorities of running animals; overcome size-related challenges. Weight-support demands lead to and (2) how do these priorities vary with terrain and body size? a shift towards straighter legs and stiffer steady gait with increasing Running animals must control their legs to balance numerous, body size, but it remains unknown whether non-steady locomotor sometimes conflicting, task-level demands including minimising priorities diverge with size. -

Nitte University Journal March 2013.Cdr
Published online: 2020-04-29 NUJHS Vol. 3, No.1, March 2013, ISSN 2249-7110 Nitte University Journal of Health Science Short Communication HOPPING IN BIRDS: IS THE CHOICE OF GAIT INFLUENCED BY CERVICAL MOBILITY AND FIELD OF VISION ? Arunachalam Kumar Professor & Head, Department of Anatomy, K.S. Hegde Medical Academy, Nitte University, Mangalore - 575 018, India. Correspondence: Arunachalam Kumar E-mail: [email protected] Abstract : One of the more intriguing questions in avian locomotion is why some birds, when on ground, choose to hop while others prefer walking. Biped gait is common to birds as well as the most evolved among mammals, man. Observations made show that, choice of gait in birds is determined by a remote factor – the range and extent of neck mobility. The wider the gamut of cervical mobility, the wider is the 'field of vision' available. Cervical movement capability is perhaps the single most deterministic factor in the bird's choice of terrestrial gait. Keywords: bipedal gait, avifauna, visual range, cervical vertebrae, hopping. Introduction : Observations & Discussion : One of the earliest bipeds to survive to modern era, are Observations on biped mobility on birds and their birds. Birds are unique in that many species among them locomotion reveal that, generally smaller birds hop while have capability of flying in air, walking on land and larger ones stride, strut or walk. Many theories and swimming in water. The talent to exploit all three mediums, hypotheses float around the word of ornithology and air, land and water for mobility makes birds occupy special kinematics on the how and why of avian locomotion. -

The First Dsungaripterid Pterosaur from the Kimmeridgian of Germany and the Biomechanics of Pterosaur Long Bones
The first dsungaripterid pterosaur from the Kimmeridgian of Germany and the biomechanics of pterosaur long bones MICHAEL FASTNACHT Fastnacht, M. 2005. The first dsungaripterid pterosaur from the Kimmeridgian of Germany and the biomechanics of pterosaur long bones. Acta Palaeontologica Polonica 50 (2): 273–288. A partial vertebral column, pelvis and femora of a newly discovered pterosaur are described. The remains from the Upper Jurassic (Kimmeridgian) of Oker (northern Germany) can be identified as belonging to the Dsungaripteridae because the cross−sections of the bones have relatively thick walls. The close resemblance in morphology to the Lower Cretaceous Dsungaripterus allows identification of the specimen as the first and oldest record of dsungaripterids in Central Europe. Fur− thermore, it is the oldest certain record of a dsungaripterid pterosaur world wide. The biomechanical characteristics of the dsungaripterid long bone construction shows that it has less resistance against bending and torsion than in non−dsungari− pteroid pterosaurs, but has greater strength against compression and local buckling. This supports former suggestions that dsungaripterids inhabited continental areas that required an active way of life including frequent take−off and landing phases. The reconstruction of the lever arms of the pelvic musculature and the mobility of the femur indicates a quadrupedal terrestrial locomotion. Key words: Reptilia, Pterosauria, Dsungaripteridae, locomotion, biomechanics, Jurassic, Germany. Michael Fastnacht [fastnach@uni−mainz.de], Palaeontologie, Institut für Geowissenschaften, Johannes Gutenberg− Universität, D−55099 Mainz, Germany. Introduction described from this site (Laven 2001; Mateus et al. 2004; Sander et al. 2004). This and further dinosaur material has In recent years, the northern part of Germany has yielded an been collected by members of the “Verein zur Förderung der increasing number of Upper Jurassic/Lower Cretaceous niedersächsischen Paläontologie e.V.”, who regularly collect archosaurian remains. -

FORELIMB LAMENESS: the GREAT IMPERSONATOR Juliette Hart, DVM, MS, CCRT, CVA Cornell University Veterinary Specialists
FORELIMB LAMENESS: THE GREAT IMPERSONATOR Juliette Hart, DVM, MS, CCRT, CVA Cornell University Veterinary Specialists. Stamford, CT Diagnosis of forelimb lameness in canine patients can often be a labor-intensive and time- consuming process, often with multiple factors being taken into account, regardless of the actual diagnosis. The dog’s age, activity level, co-morbidities, job and environment can be key players. Close examination of the dog in motion (in hospital and at home) can be helpful when determining type and degree of lameness, and may frequently assist the clinician in determining next appropriate diagnostic tests and treatment plans. This lecture will focus on differentials associated with forelimb lameness in dogs, current diagnostic tests and potential treatments available, and finally prognoses and outcomes for specific types of shoulder forelimb lameness in dogs. Lameness Evaluation The forelimb skeleton consists of the thoracic or pectoral girdle and the bones of the forelimb. The canine scapula itself is positioned close to the sagittal plane, and the humeral head is less rounded (as compared to the human head) to assist with weight bearing. The radius takes the majority of weight-bearing in the antebrachium. And, although small, the many sesamoid bones in the carpus/paw allow for biomechanically advantageous alignment of angles of insertion of tendons at their attachments.¹ While there can be tremendous variation in the sizes of the bones themselves comparing dog to dog, the literature have reported a roughly 60% body weight distribution in the thoracic limbs.² As a clinician evaluates a patient, lameness is a key element of that examination. -

Alexander 2013 Principles-Of-Animal-Locomotion.Pdf
.................................................... Principles of Animal Locomotion Principles of Animal Locomotion ..................................................... R. McNeill Alexander PRINCETON UNIVERSITY PRESS PRINCETON AND OXFORD Copyright © 2003 by Princeton University Press Published by Princeton University Press, 41 William Street, Princeton, New Jersey 08540 In the United Kingdom: Princeton University Press, 3 Market Place, Woodstock, Oxfordshire OX20 1SY All Rights Reserved Second printing, and first paperback printing, 2006 Paperback ISBN-13: 978-0-691-12634-0 Paperback ISBN-10: 0-691-12634-8 The Library of Congress has cataloged the cloth edition of this book as follows Alexander, R. McNeill. Principles of animal locomotion / R. McNeill Alexander. p. cm. Includes bibliographical references (p. ). ISBN 0-691-08678-8 (alk. paper) 1. Animal locomotion. I. Title. QP301.A2963 2002 591.47′9—dc21 2002016904 British Library Cataloging-in-Publication Data is available This book has been composed in Galliard and Bulmer Printed on acid-free paper. ∞ pup.princeton.edu Printed in the United States of America 1098765432 Contents ............................................................... PREFACE ix Chapter 1. The Best Way to Travel 1 1.1. Fitness 1 1.2. Speed 2 1.3. Acceleration and Maneuverability 2 1.4. Endurance 4 1.5. Economy of Energy 7 1.6. Stability 8 1.7. Compromises 9 1.8. Constraints 9 1.9. Optimization Theory 10 1.10. Gaits 12 Chapter 2. Muscle, the Motor 15 2.1. How Muscles Exert Force 15 2.2. Shortening and Lengthening Muscle 22 2.3. Power Output of Muscles 26 2.4. Pennation Patterns and Moment Arms 28 2.5. Power Consumption 31 2.6. Some Other Types of Muscle 34 Chapter 3. -

Biomechanics of Terrestrial Locomotion: Asymmetric Octopedal and Quadrupedal Gaits
SCUOLA DI DOTTORATO IN SCIENZE MORFOLOGICHE, FISIOLOGICHE E DELLO SPORT DIPARTIMENTO DI FISIOLOGIA UMANA DOTTORATO DI RICERCA IN FISIOLOGIA CICLO XXIV Biomechanics of terrestrial locomotion: asymmetric octopedal and quadrupedal gaits SETTORE SCIENTIFICO DISCIPLINARE BIO-09 PhD Student: Dott. Carlo M. Biancardi Matricola: R08161 Tutor: Prof. Alberto E. Minetti Coordinatore: Prof. Paolo Cavallari Anno Accademico 2010-2011 Table of Contents Abstract...................................................................................................... 5 Introduction ...............................................................................................8 Foreword.................................................................................................................. 8 Objectives .................................................................................................................8 Thesis structure........................................................................................................ 8 Terrestrial legged locomotion ..................................................................9 Introduction .............................................................................................................9 Energetics and mechanics of terrestrial legged locomotion ................................10 Limbs mechanics ..........................................................................................................10 Size differences .............................................................................................................14 -
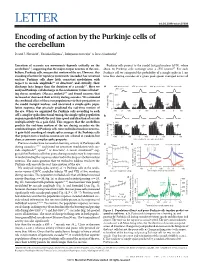
(2015) Encoding of Action by the Purkinje Cells of the Cerebellum
LETTER doi:10.1038/nature15693 Encoding of action by the Purkinje cells of the cerebellum David J. Herzfeld1, Yoshiko Kojima2, Robijanto Soetedjo2 & Reza Shadmehr1 Execution of accurate eye movements depends critically on the Purkinje cells project to the caudal fastigial nucleus (cFN), where cerebellum1–3, suggesting that the major output neurons of the cere- about 50 Purkinje cells converge onto a cFN neuron17. For each bellum, Purkinje cells, may predict motion of the eye. However, this Purkinje cell we computed the probability of a simple spike in 1-ms encoding of action for rapid eye movements (saccades) has remained time bins during saccades of a given peak speed, averaged across all unclear: Purkinje cells show little consistent modulation with respect to saccade amplitude4,5 or direction4, and critically, their discharge lasts longer than the duration of a saccade6,7.Herewe a 400° per s saccade 650° per s saccade 400° per s saccade 650° per s saccade analysed Purkinje-cell discharge in the oculomotor vermis of behav- 500° per s 8,9 200 ing rhesus monkeys (Macaca mulatta) and found neurons that N12 increased or decreased their activity during saccades. We estimated the combined effect of these two populations via their projections to 100 the caudal fastigial nucleus, and uncovered a simple-spike popu- lation response that precisely predicted the real-time motion of 0 the eye. When we organized the Purkinje cells according to each –1000 100 0 100 0 100 0 100 cell’s complex-spike directional tuning, the simple-spike population b 500° per s response predicted both the real-time speed and direction of saccade multiplicatively via a gain field.