Ecology of the Culpeo (Lycalopex Culpaeus): a Synthesis of Existing Knowledge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fur Trade and the Biotic Homogenization of Subpolar Ecosystems
Chapter 14 Fur Trade and the Biotic Homogenization of Subpolar Ecosystems Ramiro D. Crego, Ricardo Rozzi, and Jaime E. Jiménez Abstract At the southern end of the Americas exist one of the last pristine ecosys- tems in the world, the sub-Antarctic Magellanic forests ecoregion, protected by the Cape Horn Biosphere Reserve (CHBR). Despite its remote location, the CHBR has been subject to the growing infuences of globalization, a process that has driven cultural, biotic, and economic transformations in the region since the mid-twentieth century. One of the most important threats to these unique ecosystems is the increase of biological invasions. Motivated by the expanding fur industry that responded to the globalization process, American beavers (Castor canadensis), muskrats (Ondatra zibethicus), and American minks (Neovison vison) were introduced, inde- pendently, to the southern tip of South America. Research has shown that these three North American species have reassembled their native interactions to affect negatively the invaded ecosystems of the CHBR. Beavers affect river fow and R. D. Crego (*) Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA R. Rozzi Department of Philosophy and Religion and Department of Biological Sciences, University of North Texas, Denton, TX, USA Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad and Universidad de Magallanes, Punta Arenas, Chile J. E. Jiménez Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Department of Philosophy and Religion, University of North Texas, Denton, TX, USA Universidad de Magallanes, Punta Arenas, Chile © Springer Nature Switzerland AG 2018 233 R. -
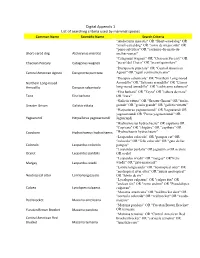
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

APROXIMACIÓN AL LENGUAJE REPERTORIAL DE LAS ESTEREOTIPIAS EN MAMIFEROS CARNIVOROS JAGUAR (Panthera Onca), GRISÓN (Galictis
APROXIMACIÓN AL LENGUAJE REPERTORIAL DE LAS ESTEREOTIPIAS EN MAMIFEROS CARNIVOROS JAGUAR (Panthera onca), GRISÓN (Galictis vittata) Y OCELOTE (Leopardus pardalis) DEL ZOOLOGICO GUATIKA, BOYACA – TIBASOSA. Laura Inés Monsalve Zuleta CÓDIGO:2014101101 Trabajo de investigación presentado como requisito parcial para optar al título de: Médica veterinaria especialista en Medicina Homeopática veterinaria Director (a): Néstor Alberto Calderón Maldonado Médico Veterinario – Universidad de la Salle Certificado en Medicina Veterinaria Homeopática – FICH “Luis G. Páez” Especialista en Bioética – Universidad El Bosque Fundación Universitaria Escuela Colombiana de Medicina Homeopática “Luis G. Páez”. Programa de Medicina Homeopática Veterinaria Bogotá, Colombia 2016 2 DEDICATORIA A mi familia, amigos y doctores que me apoyaron en esta etapa de mi vida tanto profesional como personal. 3 AGRADECIMIENTOS Agradezco a mi familia, amigos y Doctores que me ayudaron a culminar esta etapa de mi vida. 4 TABLA DE CONTENIDO: 1. RESUMEN ...................................................................................................................................... 7 2. INTRODUCCIÓN ........................................................................................................................... 8 3. JUSTIFICACIÓN ........................................................................................................................... 9 4. ESTADO DEL ARTE ................................................................................................................. -

A Review of the Ecology of the Raccoon Dog (Nyctereutes Procyonoides) in Europe
A review of the ecology of the raccoon dog (Nyctereutes procyonoides) in Europe Jaap L. Mulder De Holle Bilt 17, NL-3732 HM De Bilt, the Netherlands, e-mail: [email protected] Abstract: The raccoon dog (Nyctereutes procyonoides) was introduced from East Asia into the former USSR between 1928 and 1957. Since then it has colonised a large part of Europe and is considered an invasive alien spe- cies. This paper reviews the current knowledge on the ecology of the raccoon dog in Europe, undertaken as a basis for a risk assessment. The raccoon dog is about the size of a red fox (Vulpes vulpes). In autumn it accumulates fat and, in areas with cold winters, it may stay underground for weeks. It does not dig and often uses badger (Meles meles) setts and fox earths for reproduction. Raccoon dogs are monogamous. Each pair occupies a fixed home range the periphery of which often overlaps with that of neighbours. Pre-breeding population density usually is between 0.5 and 1.0 adults/km2. Habitat use is characterised by a preference for shores, wet habitats and deciduous forests. Foraging raccoon dogs move quite slowly, mostly staying in cover. They are omnivorous gatherers rather than hunters. Their diet is variable, with amphibians, small mammals, carrion, maize and fruits being important components. There is no proof of a negative effect on their prey populations. Raccoon dogs produce a relatively large litter of usually 6 to 9 cubs. After six weeks the den is left and the whole family roams around. From July onwards the cubs, still only half grown, start to disperse. -

Predicting Greater Grison Galictis Vittata Presence from Scarce Records in the Department of Cordoba, Colombia
ORIGINAL CONTRIBUTION Predicting Greater Grison Galictis vittata presence from scarce records in the department of Cordoba, Colombia José F. GONZÁLEZ-MAYA1*, Julio J. CHACÓN PACHECO2, Javier RACERO-CASARRUBIA2, Erika HUMANEZ-LÓPEZ2 & Andrés ARIAS-ALZATE3 1. Proyecto de Conservación de Aguas y Tierras, ProCAT Colombia/Internacional, Calle 97ª # 10-62, Of. 202, Bogotá, Abstract. Colombia. The Greater Grison, Galictis vittata, is a poorly known species in Colombia. Throughout 2. Grupo de Investigación its range major knowledge gaps exist regarding its ecology and conservation. To compile Biodiversidad Unicórdoba, and analyse information about the species´ distribution records in the department of Universidad de Córdoba, Cordoba, Colombia and assess its presence probability according to landscape attributes, Montería, Córdoba, Colombia. we conducted a literature review of all wildlife studies in the region and compiled all possible direct presence records of the species in the department. We generated random 3.Grupo de Mastozoología, location points and characterized each distribution and random location by their distance to landscape attributes and land-cover type and modelled landscape presence using a Instituto de Biología, Multiple Logistic Regression approach. We found 33 records of the species in Cordoba Universidad de Antioquía. with most of the records distributed in the subregion of Alto Sinú (36%). Higher presence Medellín, Colombia. probabilities are localized in areas near forests mostly in the southern parts of the department, showing the species is still related with the largest forest blocks. Grisons Correspondence: appears to potentially tolerate some levels of disturbance but is still dependent to forest. The influence of natural habitats and abundance across the department and other areas José F. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Vulpes Vulpes) Evolved Throughout History?
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Environmental Studies Undergraduate Student Theses Environmental Studies Program 2020 TO WHAT EXTENT HAS THE RELATIONSHIP BETWEEN HUMANS AND RED FOXES (VULPES VULPES) EVOLVED THROUGHOUT HISTORY? Abigail Misfeldt University of Nebraska-Lincoln Follow this and additional works at: https://digitalcommons.unl.edu/envstudtheses Part of the Environmental Education Commons, Natural Resources and Conservation Commons, and the Sustainability Commons Disclaimer: The following thesis was produced in the Environmental Studies Program as a student senior capstone project. Misfeldt, Abigail, "TO WHAT EXTENT HAS THE RELATIONSHIP BETWEEN HUMANS AND RED FOXES (VULPES VULPES) EVOLVED THROUGHOUT HISTORY?" (2020). Environmental Studies Undergraduate Student Theses. 283. https://digitalcommons.unl.edu/envstudtheses/283 This Article is brought to you for free and open access by the Environmental Studies Program at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Environmental Studies Undergraduate Student Theses by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TO WHAT EXTENT HAS THE RELATIONSHIP BETWEEN HUMANS AND RED FOXES (VULPES VULPES) EVOLVED THROUGHOUT HISTORY? By Abigail Misfeldt A THESIS Presented to the Faculty of The University of Nebraska-Lincoln In Partial Fulfillment of Requirements For the Degree of Bachelor of Science Major: Environmental Studies Under the Supervision of Dr. David Gosselin Lincoln, Nebraska November 2020 Abstract Red foxes are one of the few creatures able to adapt to living alongside humans as we have evolved. All humans and wildlife have some id of relationship, be it a friendly one or one of mutual hatred, or simply a neutral one. Through a systematic research review of legends, books, and journal articles, I mapped how humans and foxes have evolved together. -

Allozyme Divergence Within the Canidae
Syst. Zooi, 36(4):339-355, 1987 ALLOZYME DIVERGENCE WITHIN THE CANIDAE ROBERT K. WAYNE1 AND STEPHEN J. O'BRIEN Laboratory of Viral Carcinogenesis, Section of Genetics, National Cancer Institute, Frederick, Maryland 21701 Abstract.—Protein products of 51 genetic loci were analyzed by gel electrophoresis using extracts of blood and tissue culture specimens from 12 of the 14 extant canid genera. Genetic Downloaded from distances were calculated and used to derive phenetic trees. The results suggest that the Canidae can be divided into several distinct groups. The wolf-like canids are a group that includes species in the genus Canis and Lycaon pictus (African wild dog). Speothos venaticus (Brazilian bush dog) is weakly associated with this group. Based on the calibration of a consensus tree with a fossil date, Canis mesomelas (black-backed jackal) and Speothos venaticus separated first, approximately 6 million years before present (MYBP). Lycaon pictus and C. latrans (coyote) separated from the line leading to C. lupus (grey wolf) and C. familiaris (domestic dog) approximately 3 MYBP. These http://sysbio.oxfordjournals.org/ results suggest that the blade-like trenchant heel on the carnassial tooth has evolved indepen- dently at least twice within the Canidae. Several distinct genetic stocks appear to have led to the extant South American canids. Chrys- ocyon brachyurus (maned wolf) is estimated to have diverged from Dusicyon vetulus (hoary fox) and Cerdocyon thous (crab-eating fox) approximately 6 MYBP. The divergence time of the last two genera is fairly recent (2-3 MYBP) and is coincident with the opening of the Panamanian land bridge. -

Temporal Changes in the Diet of Two Sympatric Carnivorous Mammals in a Protected Area of South–Central Chile Affected by a Mixed–Severity Forest Fire
Animal Biodiversity and Conservation 43.2 (2020) 177 Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire A. H. Zúñiga, J. R. Rau, V. Fuenzalida, A. Fuentes–Ramírez Zúñiga, A. H., Rau, J. R., Fuenzalida, V., Fuentes–Ramírez, A., 2020. Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire. Animal Biodiversity and Conservation, 43.2: 177–186, Doi: https://doi.org/10.32800/abc.2020.43.0177 Abstract Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire. Fire is a significant disruptive agent in various ecosystems around the world. It can affect the availability of resources in a given area, modulating the interaction between competing species. We studied the diet of the culpeo fox (Lycalopex culpaeus) and cougar (Puma concolor) for two consecutive years in a protected area of southern–central Chile which was affected by a wildfire. Significant differences were observed in the dietary pattern between the two species, showing their trophic segregation. In the two years of the study, the predominant prey for cougar was an exotic species, the European hare (Lepus europaeus), implying a simplification of its trophic spectrum with respect to that reported in other latitudes. The ecological consequences related to this scenario are discussed. Key words: Dietary overlap, Predation, Post–fire dynamics, Microhabitat, Rodent cycles, Selectivity Resumen Cambios temporales en la dieta de dos mamíferos carnívoros simpátridas en una zona protegida del centro y el sur de Chile afectada por un incendio forestal de intensidad desigual. -

PICA Project Report (Action A2.2 & 2.3)
PICA Project Report (Action A2.2 & 2.3) Investigation of Pallas’s cat activity patterns and temporal interactions with sympatric species Authors: Katarzyna Ruta, Gustaf Samelius, David Barclay, Emma Nygren PICA - “Conservation of the Pallas’s cat through capacity building, research, and global planning” 1. Introduction: 1.1 Activity patterns of wild felids: Activity patterns form a part of species’ adaptation to their environment (Beltran & Delibes, 1994) and are therefore a fundamental aspect of animal behaviour (Nielsen, 1983; Weller & Bennett, 2001). Felids are generally considered to be crepuscular and nocturnal in their activity (Kitchener, 1991), although they are well adapted to function in a wide range of light conditions (Sunquist & Sunquist, 2002). Numerous abiotic pressures and biotic interactions are known to shape the temporal behaviour of (cat-like) carnivores (Marinho et al., 2018), including changes in temperature (Beltran & Delibes, 1994; Podolski et al., 2013), light (Huck et al., 2017; Heurich et al., 2014) and season (Podolski et al., 2013; Manfredi et al., 2011), sex and reproductive status of the animal (Kolbe & Squires, 2007; Schmidt, 1999; Schmidt et al., 2009), predation risk (Caro, 2005; Farías et al., 2012) and human disturbance (Wolf & Ale, 2009; Ale & Brown, 2009). Owing to the dietary constraints of carnivores whose preys have their own well-defined circadian rhythms (Halle, 2000; Zielinski, 2000), the availability and vulnerability of prey is, however, considered as one of the main influences on predator temporal activity (Zielinski, 1988; Lodé, 1995). According to Optimal Foraging Theory, predators are expected to synchronize their daily activity with the activity of their most profitable prey, increasing the probability of encounters while reducing energy expenditure (MacArthur & Pianka, 1966; Monterroso et al., 2013; Emmons, 1987). -

La Brea and Beyond: the Paleontology of Asphalt-Preserved Biotas
La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas Edited by John M. Harris Natural History Museum of Los Angeles County Science Series 42 September 15, 2015 Cover Illustration: Pit 91 in 1915 An asphaltic bone mass in Pit 91 was discovered and exposed by the Los Angeles County Museum of History, Science and Art in the summer of 1915. The Los Angeles County Museum of Natural History resumed excavation at this site in 1969. Retrieval of the “microfossils” from the asphaltic matrix has yielded a wealth of insect, mollusk, and plant remains, more than doubling the number of species recovered by earlier excavations. Today, the current excavation site is 900 square feet in extent, yielding fossils that range in age from about 15,000 to about 42,000 radiocarbon years. Natural History Museum of Los Angeles County Archives, RLB 347. LA BREA AND BEYOND: THE PALEONTOLOGY OF ASPHALT-PRESERVED BIOTAS Edited By John M. Harris NO. 42 SCIENCE SERIES NATURAL HISTORY MUSEUM OF LOS ANGELES COUNTY SCIENTIFIC PUBLICATIONS COMMITTEE Luis M. Chiappe, Vice President for Research and Collections John M. Harris, Committee Chairman Joel W. Martin Gregory Pauly Christine Thacker Xiaoming Wang K. Victoria Brown, Managing Editor Go Online to www.nhm.org/scholarlypublications for open access to volumes of Science Series and Contributions in Science. Natural History Museum of Los Angeles County Los Angeles, California 90007 ISSN 1-891276-27-1 Published on September 15, 2015 Printed at Allen Press, Inc., Lawrence, Kansas PREFACE Rancho La Brea was a Mexican land grant Basin during the Late Pleistocene—sagebrush located to the west of El Pueblo de Nuestra scrub dotted with groves of oak and juniper with Sen˜ora la Reina de los A´ ngeles del Rı´ode riparian woodland along the major stream courses Porciu´ncula, now better known as downtown and with chaparral vegetation on the surrounding Los Angeles. -

Species-Specific Responses of Carnivores to Human-Induced Landscape Changes in Central Argentina
RESEARCH ARTICLE Species-Specific Responses of Carnivores to Human-Induced Landscape Changes in Central Argentina Nicolás Caruso1,2*, Mauro Lucherini1,2, Daniel Fortin3, Emma B. Casanave1,4 1 Grupo de Ecología Comportamental de Mamíferos (GECM), Cátedra de Fisiología Animal, Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur, Bahía Blanca, Argentina, 2 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina, 3 Département de biologie and Centre d'étude de la forêt, Département de Biologie, Université Laval, Québec, Canada, 4 Instituto de Ciencias Biológicas y Biomédicas del Sur (INBIOSUR), Universidad Nacional del Sur and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Bahía Blanca, Argentina * [email protected] Abstract OPEN ACCESS The role that mammalian carnivores play in ecosystems can be deeply altered by human- driven habitat disturbance. While most carnivore species are negatively affected, the impact Citation: Caruso N, Lucherini M, Fortin D, Casanave of habitat changes is expected to depend on their ecological flexibility. We aimed to identify EB (2016) Species-Specific Responses of Carnivores to Human-Induced Landscape Changes in Central key factors affecting the habitat use by four sympatric carnivore species in landscapes of Argentina. PLoS ONE 11(3): e0150488. doi:10.1371/ central Argentina. Camera trapping surveys were carried out at 49 sites from 2011 to 2013. journal.pone.0150488 Each site was characterized by 12 habitat attributes, including human disturbance and frag- Editor: Nei Moreira, Federal University of Parana mentation. Four landscape gradients were created from Principal Component Analysis and – (UFPR) Campus Palotina, BRAZIL their influence on species-specific habitat use was studied using Generalized Linear Mod- Received: October 27, 2015 els.