MECHANICS and SELECTIVITY of FILTRATION by TUNICATES By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
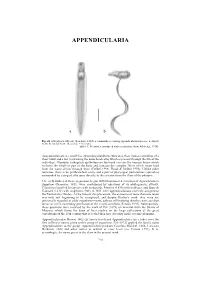
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Brains of Primitive Chordates 439
Brains of Primitive Chordates 439 Brains of Primitive Chordates J C Glover, University of Oslo, Oslo, Norway although providing a more direct link to the evolu- B Fritzsch, Creighton University, Omaha, NE, USA tionary clock, is nevertheless hampered by differing ã 2009 Elsevier Ltd. All rights reserved. rates of evolution, both among species and among genes, and a still largely deficient fossil record. Until recently, it was widely accepted, both on morpholog- ical and molecular grounds, that cephalochordates Introduction and craniates were sister taxons, with urochordates Craniates (which include the sister taxa vertebrata being more distant craniate relatives and with hemi- and hyperotreti, or hagfishes) represent the most chordates being more closely related to echinoderms complex organisms in the chordate phylum, particu- (Figure 1(a)). The molecular data only weakly sup- larly with respect to the organization and function of ported a coherent chordate taxon, however, indicat- the central nervous system. How brain complexity ing that apparent morphological similarities among has arisen during evolution is one of the most chordates are imposed on deep divisions among the fascinating questions facing modern science, and it extant deuterostome taxa. Recent analysis of a sub- speaks directly to the more philosophical question stantially larger number of genes has reversed the of what makes us human. Considerable interest has positions of cephalochordates and urochordates, pro- therefore been directed toward understanding the moting the latter to the most closely related craniate genetic and developmental underpinnings of nervous relatives (Figure 1(b)). system organization in our more ‘primitive’ chordate relatives, in the search for the origins of the vertebrate Comparative Appearance of Brains, brain in a common chordate ancestor. -

Tunicate Mitogenomics and Phylogenetics: Peculiarities of the Herdmania Momus Mitochondrial Genome and Support for the New Chordate Phylogeny
Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny. Tiratha Raj Singh, Georgia Tsagkogeorga, Frédéric Delsuc, Samuel Blanquart, Noa Shenkar, Yossi Loya, Emmanuel Douzery, Dorothée Huchon To cite this version: Tiratha Raj Singh, Georgia Tsagkogeorga, Frédéric Delsuc, Samuel Blanquart, Noa Shenkar, et al.. Tu- nicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny.. BMC Genomics, BioMed Central, 2009, 10, pp.534. 10.1186/1471-2164-10-534. halsde-00438100 HAL Id: halsde-00438100 https://hal.archives-ouvertes.fr/halsde-00438100 Submitted on 2 Dec 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. BMC Genomics BioMed Central Research article Open Access Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny Tiratha Raj Singh†1, Georgia Tsagkogeorga†2, Frédéric Delsuc2, Samuel Blanquart3, Noa -

Ascidiacea (Chordata: Tunicata) of Greece: an Updated Checklist
Biodiversity Data Journal 4: e9273 doi: 10.3897/BDJ.4.e9273 Taxonomic Paper Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist Chryssanthi Antoniadou‡, Vasilis Gerovasileiou§§, Nicolas Bailly ‡ Department of Zoology, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece § Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research, Heraklion, Greece Corresponding author: Chryssanthi Antoniadou ([email protected]) Academic editor: Christos Arvanitidis Received: 18 May 2016 | Accepted: 17 Jul 2016 | Published: 01 Nov 2016 Citation: Antoniadou C, Gerovasileiou V, Bailly N (2016) Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist. Biodiversity Data Journal 4: e9273. https://doi.org/10.3897/BDJ.4.e9273 Abstract Background The checklist of the ascidian fauna (Tunicata: Ascidiacea) of Greece was compiled within the framework of the Greek Taxon Information System (GTIS), an application of the LifeWatchGreece Research Infrastructure (ESFRI) aiming to produce a complete checklist of species recorded from Greece. This checklist was constructed by updating an existing one with the inclusion of recently published records. All the reported species from Greek waters were taxonomically revised and cross-checked with the Ascidiacea World Database. New information The updated checklist of the class Ascidiacea of Greece comprises 75 species, classified in 33 genera, 12 families, and 3 orders. In total, 8 species have been added to the previous species list (4 Aplousobranchia, 2 Phlebobranchia, and 2 Stolidobranchia). Aplousobranchia was the most speciose order, followed by Stolidobranchia. Most species belonged to the families Didemnidae, Polyclinidae, Pyuridae, Ascidiidae, and Styelidae; these 4 families comprise 76% of the Greek ascidian species richness. The present effort revealed the limited taxonomic research effort devoted to the ascidian fauna of Greece, © Antoniadou C et al. -

Ascidian News #87 June 2021
ASCIDIAN NEWS* Gretchen Lambert 12001 11th Ave. NW, Seattle, WA 98177 206-365-3734 [email protected] home page: http://depts.washington.edu/ascidian/ Number 87 June 2021 Well, here we are still in this pandemic! I asked how you all are and again received many responses. A number are included in the next two sections. Nearly everyone still expresses confidence at having met the challenges and a great feeling of accomplishment even though tired of the whole thing; congratulations to you all! There are 117 new publications since December! Thanks to so many for the contributions and for letting me know how important AN continues to be. Please keep in touch and continue to send me contributions for the next issue. Keep safe, keep working, and good luck to everyone. *Ascidian News is not part of the scientific literature and should not be cited as such. NEWS AND VIEWS 1. From Hiroki Nishida ([email protected]) : In Japan, we are very slow to be vaccinated, but the labs are ordinarily opened and we can continue working. Number of patients are gradually increasing though and we are waiting for vaccines. I have to stay in my home and the lab. Postponement of 11th ITM (International Tunicate Meeting) This is an announcement about 11th ITM that had been planned to be held in July 2021 in Kobe, Japan. It is postponed by a year because of the global spread of COVID-19. We had an 11th ITM board meeting, and came to the conclusion that we had to reschedule it for July 2022 at the same venue (Konan University, Kobe, Japan) and similar dates (July 11 to 16). -

Spatial Distribution Patterns of Appendicularians in the Drake Passage: Potential Indicators of Water Masses?
diversity Article Spatial Distribution Patterns of Appendicularians in the Drake Passage: Potential Indicators of Water Masses? Marcin Kalarus 1 and Anna Panasiuk 2,* 1 Department of Aquatic Ecology, Maritime Institute, Gdynia Maritime University, Smoluchowskiego 1/3, 80-214 Gda´nsk,Poland; [email protected] 2 Department of Marine Plankton Research, Faculty of Oceanography and Geography, Institute of Oceanography, University of Gda´nsk,Av. Marszałka J. Piłsudskiego 46, 81-378 Gdynia, Poland * Correspondence: [email protected]; Tel.: +485-8523-6844 Abstract: Appendicularians are one of the most common animals found within zooplankton as- semblages. They play a very important role as filter feeders but are, unfortunately, inconsistently reported in the Antarctic literature. The present paper attempts to describe the zonal diversity of appendicularians and the main environmental factors influencing their communities in the Drake Passage. Samples were collected during Antarctic summer in 2009–2010. A total of eight species of larvaceans were identified. Fritillaria borealis was the species found in the highest numbers in almost the entire studied area, and was observed at all sampling stations. The distributions of other taxa were limited to specific hydrological zones and hydrological conditions. F. fraudax and Oikopleura gaussica were typical of the areas between the Polar Front and the Subantarctic Front zones, and their distributions were significantly correlated with temperature and salinity, likely making them good indicator species. The F. fusiformis distribution was strictly related to South American waters. In summary, temperature was the strongest environmental factor influencing the larvacean community structure in the Drake Passage, and we also found that testing environmental factors on larvaceans Citation: Kalarus, M.; Panasiuk, A. -

A Chordate Species Lacking Nodal Utilizes Calcium Oscillation and Bmp for Left–Right Patterning
A chordate species lacking Nodal utilizes calcium oscillation and Bmp for left–right patterning Takeshi A. Onumaa,1, Momoko Hayashia, Fuki Gyojab, Kanae Kishia, Kai Wanga, and Hiroki Nishidaa aDepartment of Biological Sciences, Graduate School of Science, Osaka University, 560-0043 Toyonaka, Osaka, Japan; and bMarine Genomics Unit, Okinawa Institute of Science and Technology Graduate University, 904-0495 Onna, Okinawa, Japan Edited by Edward M. De Robertis, David Geffen School of Medicine, University of California, Los Angeles, CA, and approved December 20, 2019 (received for review September 27, 2019) Larvaceans are chordates with a tadpole-like morphology. In neurula by ciliary movement (27, 28). These are considered to contrast to most chordates of which early embryonic morphology is be biological cues that induce the left-sided expression of Nodal,a bilaterally symmetric and the left–right (L–R) axis is specified by the member of the TGF-β family, leading to the L–R asymmetry of Nodal pathway later on, invariant L–R asymmetry emerges in four- Deuterostomia (e.g., vertebrates) and Lophotrochozoa (e.g., pond cell embryos of larvaceans. The asymmetric cell arrangements exist snails). However, these mechanisms are not universal. For ex- through development of the tailbud. The tail thus twists 90° in a ample, motile cilia are not associated with L–R patterning in the counterclockwise direction relative to the trunk, and the tail nerve pond snail (29, 30), and Nodal is lost in Ecdysozoa (e.g., nem- cord localizes on the left side. Here, we demonstrate that larvacean atodes and flies). This diversity of symmetry-breaking mecha- embryos have nonconventional L–R asymmetries: 1) L- and R-cells of nisms suggests that there may be organisms with novel strategies 2+ the two-cell embryo had remarkably asymmetric cell fates; 2) Ca for L–R patterning. -

Ascidian News #82 December 2018
ASCIDIAN NEWS* Gretchen Lambert 12001 11th Ave. NW, Seattle, WA 98177 206-365-3734 [email protected] home page: http://depts.washington.edu/ascidian/ Number 82 December 2018 A big thank-you to all who sent in contributions. There are 85 New Publications listed at the end of this issue. Please continue to send me articles, and your new papers, to be included in the June 2019 issue of AN. It’s never too soon to plan ahead. *Ascidian News is not part of the scientific literature and should not be cited as such. NEWS AND VIEWS 1. From Stefano Tiozzo ([email protected]) and Remi Dumollard ([email protected]): The 10th Intl. Tunicata Meeting will be held at the citadel of Saint Helme in Villefranche sur Mer (France), 8- 12 July 2019. The web site with all the information will be soon available, save the date! We are looking forward to seeing you here in the Riviera. A bientôt! Remi and Stefano 2. The 10th Intl. Conference on Marine Bioinvasions was held in Puerto Madryn, Patagonia, Argentina, October 16-18. At the conference website (http://www.marinebioinvasions.info/index) the program and abstracts in pdf can be downloaded. Dr. Rosana Rocha presented one of the keynote talks: "Ascidians in the anthropocene - invasions waiting to happen". See below under Meetings Abstracts for all the ascidian abstracts; my thanks to Evangelina Schwindt for compiling them. The next (11th) meeting will be in Annapolis, Maryland, organized by Greg Ruiz, Smithsonian Invasions lab, date to be determined. 3. Conference proceedings of the May 2018 Invasive Sea Squirt Conference will be peer reviewed and published in a special issue of the REABIC journal Management of Biological Invasions. -

Abstracts (In Speaking Order; As Listed in Program Schedule)
Abstracts (in speaking order; as listed in program schedule) A remarkable case of non-invasion in British Columbia, Canada Gretchen Lambert Univ. of Washington Friday Harbor Laboratories, Friday Harbor WA 98177 Between July 21-August 11, 2017, a three-week comprehensive marine biodiversity survey was carried out at a small remote region of the central British Columbia coast at and near the Calvert Island Marine Station (Hakai Institute). There is no commercial shipping to this area and only a small amount of recreational-size boat traffic. The survey included daily sampling by the staff and a number of visiting taxonomists with specialties covering all the major groups of invertebrates. Many marine habitats were sampled: rocky and sand/gravel intertidal, eelgrass meadows, shallow and deeper subtidal by snorkel and scuba, plus artificial surfaces of settlement plates set out up to a year ago, and the sides and bottom of the large floating dock at the Institute. Many new species were recorded by all the taxonomists; in this limited remote area I identified 37 ascidian species, including 3 new species, which represents almost 1/3 of all the known North American species from Alaska to southern California. Remarkably, only one is a possible non-native, Diplosoma listerianum, and it was collected mostly on natural substrates including deeper areas sampled by scuba; one colony occurred on a settlement plate. There were no botryllids, no Styela clava, no Didemnum vexillum, though these are all common non-natives in other parts of BC and the entire U.S. west coast. Most of the species are the same as in northern California, Washington, and southern BC, with only a small overlap of a few of the known Alaska spp. -

Unique Crystallographic Pattern in the Macro to Atomic Structure Of
Journal of Structural Biology 183 (2013) 191–198 Contents lists available at SciVerse ScienceDirect Journal of Structural Biology journal homepage: www.elsevier.com/locate/yjsbi Unique crystallographic pattern in the macro to atomic structure of Herdmania momus vateritic spicules ⇑ Lee Kabalah-Amitai a, Boaz Mayzel c, Paul Zaslansky d, Yaron Kauffmann a, Peter Clotens e, Boaz Pokroy a,b, a Department of Materials Science and Engineering, Technion – Israel Institute of Technology, 32000 Haifa, Israel b Russell Berrie Nanotechnology Institute, Technion – Israel Institute of Technology, 32000 Haifa, Israel c Department of Biology, Tel Aviv University, 69978 Tel Aviv, Israel d Berlin – Brandenburg Center for Regenerative Therapies, Julius Wolff Institut, Charité – Universitätsmedizin, Berlin, Germany e European Synchrotron Radiation Facility, BP 220, F-38043 Grenoble Cedex, France article info abstract Article history: Biogenic vaterite is extremely rare. The only known example of a completely vateritic mineralized struc- Available online 10 May 2013 ture is the spicule of the solitary ascidian, Herdmania momus. In characterizing the structure of these spic- ules, using state-of-the-art techniques such as synchrotron X-ray diffraction and synchrotron micro- and Keywords: nanotomography, we observed a continuous structural pattern from the macro down to the micro, nano, Biomineralization and atomic scales. We show that the spicules demonstrate a unique architecture composed of micron- Vaterite sized, hexagonally faceted thorns organized in partial spirals along the cylinder-like polycrystalline body Biogenic crystals of the spicule, and tilted from it at an angle of about 26°. This morphological orientation coincides with High-resolution characterization techniques the crystallographic orientation relationship between each thorn and the polycrystals within the spicule. -

Oikopleura Dioica
To: [email protected] o ABSTRACT We propose that a BAC library should be constructed for the larvacean urochordate Oikopleura dioica. This animal may be the most similar among extant animals to the last common ancestor of all chordates, and thus, its investigation can illuminate the mechanisms causing the vertebrate genome expansion, the principles that guide the evolution of functions of duplicate genes in development, and the origin of vertebrate developmental innovations. Oikopleura dioica has a genome of about 70 Mb, the smallest of any chordate. o IMPORTANCE OF THE ORGANISM TO BIOMEDICAL OR BIOLOGICAL RESEARCH. The Animal. Oikopleura dioica is a 3 mm long urochordate, an abundant filter-feeder in marine plankton. Oikopleura and other members of its phylogenetic class, the larvaceans (or appendicularians) remain free swimming and tadpole-like into sexual maturity. Because larvaceans are basally diverging among the urochordates, this free-swimming life style may have been ancestral (Wada 1998). Larvaceans have more functional organ systems than do ascidian larvae; their embryos, tadpole larvae, and adults are transparent, which facilitates observations on all aspects of development (Fig. 1); embryonic development takes only 14 hours; and the generation time is less than two weeks. Furthermore, Oikopleura dioica is readily available from plankton tows for much of the year off Northern hemisphere coasts. Oikopleura dioica can be among the most abundant animals in offshore waters, even more than copepods, amounting to several animals per liter (Dagg & Ortner 1994; Capitano & Esnal 1998). With Oikopleura, one can perform single-pair matings, and culture the offspring in the lab continuously in the presence of fresh sea water (Fenaux & Gorsky, 1983; Bassham, unpubl). -

Strategic Research Fund for the Marine Environment (SRFME) Is a 6-Year (2001-2006), $20 Million Joint Venture Between CSIRO and the Western Australia Government
Strategic Research Fund for the Marine Environment (SRFME) Interim final report June 2005 Edited by John K. Keesing and John N. Heine CSIRO Marine and Atmospheric Research Strategic Research Fund for the Marine Environment: interim final report, June 2005. Bibliography. ISBN 1 921061 06 5. 1. CSIRO – Appropriations and expenditures. 2. Marine sciences – Research – Western Australia – Finance. 3. Marine resources – Research – Western Australia – Finance. I. Keesing, John K. II. Heine, John N. III. CSIRO. Marine and Atmospheric Research. 354.5709941 CONTENTS EXECUTIVE SUMMARY 6 CHAPTER 1 8 1.1 About SRFME 8 1.1.1 Role and Purpose of SRFME 8 1.1.2 Background to SRFME 8 1.2 Structure and Governance of SRFME 9 1.2.1 SRFME Joint Venture Management Committee 9 1.2.2 SRFME Technical Advisory Committee 10 1.2.3 SRFME Research Director and Project Leaders 10 1.3 The SRFME Framework and Research Portfolio Structure 11 1.3.1 The SRFME Framework 11 1.3.2 SRFME Research Portfolio Structure 12 1.4 Collaborative Linkages Program 13 1.4.1 SRFME PhD Scholarships 13 1.4.2 SRFME Collaborative Research Projects 13 1.4.3 State Linkage Projects 13 1.5 SRFME Core Projects 14 1.5.1 Development of the SRFME Core Projects 14 1.5.2 Biophysical Oceanography Core Project 14 1.5.3 Coastal Ecosystems and Biodiversity Core Project 14 1.5.4 Integrated Modelling Core Project 14 CHAPTER 2 15 2. COLLABORATIVE LINKAGES PROGRAM: PhD PROJECTS 15 2.1 SRFME PhD Scholarship Program 15 2.2 SRFME PhD Projects, Students, and Affi liations 15 2.3 PhD Scholars Reports 16 2.3.1 The Development