Ascidian News #82 December 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
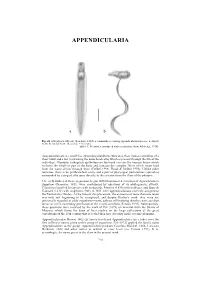
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Grebmeier, Jacqueline M., Lee W.Cooper, and Michael J
Limnol. Oceanogr., 35(S), 1990, 1 182-1195 0 1990, by the American Society of Limnology and Oceanography, Inc. Oxygen isotopic composition of bottom seawater and tunicate cellulose used as indicators of water masses in the northern Bering and Chukchi Seas Abstract -Oxygen isotopic composition of rivers, evaporated surface ocean waters, bottom seawater and tunicate cellulose were used melting glaciers, and melting sea ice can be as short-term and long-term indicators, respec- tively, of water-mass characteristics in the north- separated and water types characterized (e.g. ern Bering and Chukchi Seas. Oxygen isotopic Epstein and Mayeda 1953; Tan and Strain composition of northeastern Bering Sea waters is 1980; Bedard et al. 198 1). In contrast to the influenced by Yukon River inflows of IsO-de- variability in the surface ocean, average 180 : pleted continental water mixing with relatively 180-enriched waters contributed by the Anadyr 160 ratios for the deep (> 500 m) sea vary Current. Tunicate cellulose sampled under Alas- by < 1%~ when expressed in the conven- ka coastal water is more depleted in IsO than that tional 6 notation: collected under Bering shelf and Anadyr waters, which reflects the oxygen isotopic composition 6180 = (Rstd/R,mple- 1) X 1030/oo (1) of these waters. Tunicate cellulose collected un- der the mixed Bering shelf water displays inter- where R = 180 : l 6O and std is Standard Mean mediate 6180 values. Oxygen isotopic analyses of Ocean Water (SMOW). The low variability bottom seawater were used to determine the spa- in V80 values of waters in the deep sea has tial location and influence of continental and led to widespread use of oxygen isotopes as coastal-derived precipitation and of sea-ice for- mation on water-mass structure on the continen- a paleothermometric indicator. -

Brains of Primitive Chordates 439
Brains of Primitive Chordates 439 Brains of Primitive Chordates J C Glover, University of Oslo, Oslo, Norway although providing a more direct link to the evolu- B Fritzsch, Creighton University, Omaha, NE, USA tionary clock, is nevertheless hampered by differing ã 2009 Elsevier Ltd. All rights reserved. rates of evolution, both among species and among genes, and a still largely deficient fossil record. Until recently, it was widely accepted, both on morpholog- ical and molecular grounds, that cephalochordates Introduction and craniates were sister taxons, with urochordates Craniates (which include the sister taxa vertebrata being more distant craniate relatives and with hemi- and hyperotreti, or hagfishes) represent the most chordates being more closely related to echinoderms complex organisms in the chordate phylum, particu- (Figure 1(a)). The molecular data only weakly sup- larly with respect to the organization and function of ported a coherent chordate taxon, however, indicat- the central nervous system. How brain complexity ing that apparent morphological similarities among has arisen during evolution is one of the most chordates are imposed on deep divisions among the fascinating questions facing modern science, and it extant deuterostome taxa. Recent analysis of a sub- speaks directly to the more philosophical question stantially larger number of genes has reversed the of what makes us human. Considerable interest has positions of cephalochordates and urochordates, pro- therefore been directed toward understanding the moting the latter to the most closely related craniate genetic and developmental underpinnings of nervous relatives (Figure 1(b)). system organization in our more ‘primitive’ chordate relatives, in the search for the origins of the vertebrate Comparative Appearance of Brains, brain in a common chordate ancestor. -

Temperature and Salinity Sensitivity of the Invasive Ascidian Microcosmus Exasperatus Heller, 1878
Aquatic Invasions (2016) Volume 11, Issue 1: 33–43 DOI: http://dx.doi.org/10.3391/ai.2016.11.1.04 Open Access © 2016 The Author(s). Journal compilation © 2016 REABIC Research Article Temperature and salinity sensitivity of the invasive ascidian Microcosmus exasperatus Heller, 1878 1 1,2 Lilach Raijman Nagar and Noa Shenkar * 1Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, Tel Aviv, 69978, Israel 2The Steinhardt Museum of Natural History and National Research Center, Tel Aviv University, Tel Aviv, Israel *Corresponding author E-mail: [email protected] Received: 5 May 2015 / Accepted: 24 November 2015 / Published online: 30 December 2015 Handling editor: Vadim Panov Abstract Environmental factors, such as temperature and salinity, are known to influence distribution patterns and invasion success in ascidians. The solitary ascidian Microcosmus exasperatus Heller, 1878 has a wide global distribution and can be found in both tropical and sub-tropical waters. In the Mediterranean Sea, it is considered to be an invasive species introduced through the Suez Canal, with a restricted distribution in the eastern Mediterranean. Despite its global distribution, the environmental tolerances of this species are poorly known. We examined the effect of varying temperature and salinity on the survival of adult individuals of M. exasperatus in a laboratory setting to partially determine its environmental tolerance range. In addition, it’s global and local distribution as well as the seasonal abundance in ‘Akko Bay (northern Mediterranean coast of Israel) were examined. Field observations and laboratory experiments show that M. exasperatus is able to tolerate a temperature range of 12–30 ºC, and salinity of 37–45, but it survived poorly in salinity of 33–35 and temperatures > 32 ºC. -

Life-History Strategies of a Native Marine Invertebrate Increasingly Exposed to Urbanisation and Invasion
Temporal Currency: Life-history strategies of a native marine invertebrate increasingly exposed to urbanisation and invasion A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Zoology University of Canterbury New Zealand Jason Suwandy 2012 Contents List of Figures ......................................................................................................................................... iii List of Tables .......................................................................................................................................... vi Acknowledgements ............................................................................................................................... vii Abstract ................................................................................................................................................ viii CHAPTER ONE - General Introduction .................................................................................................... 1 1.1 Marine urbanisation and invasion ................................................................................................ 2 1.2 Successful invasion and establishment of populations ................................................................ 4 1.3 Ascidians ....................................................................................................................................... 7 1.4 Native ascidians as study organisms ............................................................................................ -

Halocynthia Roretzi
Sekigami et al. Zoological Letters (2017) 3:17 DOI 10.1186/s40851-017-0078-3 RESEARCH ARTICLE Open Access Hox gene cluster of the ascidian, Halocynthia roretzi, reveals multiple ancient steps of cluster disintegration during ascidian evolution Yuka Sekigami1, Takuya Kobayashi1, Ai Omi1, Koki Nishitsuji2, Tetsuro Ikuta1, Asao Fujiyama3, Noriyuki Satoh2 and Hidetoshi Saiga1* Abstract Background: Hox gene clusters with at least 13 paralog group (PG) members are common in vertebrate genomes and in that of amphioxus. Ascidians, which belong to the subphylum Tunicata (Urochordata), are phylogenetically positioned between vertebrates and amphioxus, and traditionally divided into two groups: the Pleurogona and the Enterogona. An enterogonan ascidian, Ciona intestinalis (Ci), possesses nine Hox genes localized on two chromosomes; thus, the Hox gene cluster is disintegrated. We investigated the Hox gene cluster of a pleurogonan ascidian, Halocynthia roretzi (Hr) to investigate whether Hox gene cluster disintegration is common among ascidians, and if so, how such disintegration occurred during ascidian or tunicate evolution. Results: Our phylogenetic analysis reveals that the Hr Hox gene complement comprises nine members, including one with a relatively divergent Hox homeodomain sequence. Eight of nine Hr Hox genes were orthologous to Ci-Hox1, 2, 3, 4, 5, 10, 12 and 13. Following the phylogenetic classification into 13 PGs, we designated Hr Hox genes as Hox1, 2, 3, 4, 5, 10, 11/12/13.a, 11/12/13.b and HoxX. To address the chromosomal arrangement of the nine Hox genes, we performed two-color chromosomal fluorescent in situ hybridization, which revealed that the nine Hox genes are localized on a single chromosome in Hr, distinct from their arrangement in Ci. -

1471-2148-9-187.Pdf
BMC Evolutionary Biology BioMed Central Research article Open Access An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models Georgia Tsagkogeorga1,2, Xavier Turon3, Russell R Hopcroft4, Marie- Ka Tilak1,2, Tamar Feldstein5, Noa Shenkar5,6, Yossi Loya5, Dorothée Huchon5, Emmanuel JP Douzery1,2 and Frédéric Delsuc*1,2 Address: 1Université Montpellier 2, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 2CNRS, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 3Centre d'Estudis Avançats de Blanes (CEAB, CSIC), Accés Cala S. Francesc 14, 17300 Blanes (Girona), Spain, 4Institute of Marine Science, University of Alaska Fairbanks, Fairbanks, Alaska, USA, 5Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, 69978, Israel and 6Department of Biology, University of Washington, Seattle WA 98195, USA Email: Georgia Tsagkogeorga - [email protected]; Xavier Turon - [email protected]; Russell R Hopcroft - [email protected]; Marie-Ka Tilak - [email protected]; Tamar Feldstein - [email protected]; Noa Shenkar - [email protected]; Yossi Loya - [email protected]; Dorothée Huchon - [email protected]; Emmanuel JP Douzery - [email protected]; Frédéric Delsuc* - [email protected] * Corresponding author Published: 5 August 2009 Received: 16 October 2008 Accepted: 5 August 2009 BMC Evolutionary Biology 2009, 9:187 doi:10.1186/1471-2148-9-187 This article is available from: http://www.biomedcentral.com/1471-2148/9/187 © 2009 Tsagkogeorga et al; licensee BioMed Central Ltd. -

Tunicate Mitogenomics and Phylogenetics: Peculiarities of the Herdmania Momus Mitochondrial Genome and Support for the New Chordate Phylogeny
Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny. Tiratha Raj Singh, Georgia Tsagkogeorga, Frédéric Delsuc, Samuel Blanquart, Noa Shenkar, Yossi Loya, Emmanuel Douzery, Dorothée Huchon To cite this version: Tiratha Raj Singh, Georgia Tsagkogeorga, Frédéric Delsuc, Samuel Blanquart, Noa Shenkar, et al.. Tu- nicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny.. BMC Genomics, BioMed Central, 2009, 10, pp.534. 10.1186/1471-2164-10-534. halsde-00438100 HAL Id: halsde-00438100 https://hal.archives-ouvertes.fr/halsde-00438100 Submitted on 2 Dec 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. BMC Genomics BioMed Central Research article Open Access Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny Tiratha Raj Singh†1, Georgia Tsagkogeorga†2, Frédéric Delsuc2, Samuel Blanquart3, Noa -

Marine Biology
Marine Biology Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska. --Manuscript Draft-- Manuscript Number: MABI-D-16-00297 Full Title: Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska. Article Type: S.I. : Invasive Species Keywords: ascidians, biofouling, biogeography, marine invasions, nonindigenous, non-native species, North America Corresponding Author: Christina Simkanin, Phd Smithsonian Environmental Research Center Edgewater, MD UNITED STATES Corresponding Author Secondary Information: Corresponding Author's Institution: Smithsonian Environmental Research Center Corresponding Author's Secondary Institution: First Author: Christina Simkanin, Phd First Author Secondary Information: Order of Authors: Christina Simkanin, Phd Paul W. Fofonoff Kristen Larson Gretchen Lambert Jennifer Dijkstra Gregory M. Ruiz Order of Authors Secondary Information: Funding Information: California Department of Fish and Wildlife Dr. Gregory M. Ruiz National Sea Grant Program Dr. Gregory M. Ruiz Prince William Sound Regional Citizens' Dr. Gregory M. Ruiz Advisory Council Smithsonian Institution Dr. Gregory M. Ruiz United States Coast Guard Dr. Gregory M. Ruiz United States Department of Defense Dr. Gregory M. Ruiz Legacy Program Abstract: SSpecies introductions have increased dramatically in number, rate, and magnitude of impact in recent decades. In marine systems, invertebrates are the largest and most diverse component of coastal invasions throughout the world. Ascidians are conspicuous and well-studied members of this group, however, much of what is known about their invasion history is limited to particular species or locations. Here, we provide a large-scale assessment of invasions, using an extensive literature review and standardized field surveys, to characterize the invasion dynamics of non-native ascidians in the continental United States and Alaska. -

Ascidiacea (Chordata: Tunicata) of Greece: an Updated Checklist
Biodiversity Data Journal 4: e9273 doi: 10.3897/BDJ.4.e9273 Taxonomic Paper Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist Chryssanthi Antoniadou‡, Vasilis Gerovasileiou§§, Nicolas Bailly ‡ Department of Zoology, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece § Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research, Heraklion, Greece Corresponding author: Chryssanthi Antoniadou ([email protected]) Academic editor: Christos Arvanitidis Received: 18 May 2016 | Accepted: 17 Jul 2016 | Published: 01 Nov 2016 Citation: Antoniadou C, Gerovasileiou V, Bailly N (2016) Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist. Biodiversity Data Journal 4: e9273. https://doi.org/10.3897/BDJ.4.e9273 Abstract Background The checklist of the ascidian fauna (Tunicata: Ascidiacea) of Greece was compiled within the framework of the Greek Taxon Information System (GTIS), an application of the LifeWatchGreece Research Infrastructure (ESFRI) aiming to produce a complete checklist of species recorded from Greece. This checklist was constructed by updating an existing one with the inclusion of recently published records. All the reported species from Greek waters were taxonomically revised and cross-checked with the Ascidiacea World Database. New information The updated checklist of the class Ascidiacea of Greece comprises 75 species, classified in 33 genera, 12 families, and 3 orders. In total, 8 species have been added to the previous species list (4 Aplousobranchia, 2 Phlebobranchia, and 2 Stolidobranchia). Aplousobranchia was the most speciose order, followed by Stolidobranchia. Most species belonged to the families Didemnidae, Polyclinidae, Pyuridae, Ascidiidae, and Styelidae; these 4 families comprise 76% of the Greek ascidian species richness. The present effort revealed the limited taxonomic research effort devoted to the ascidian fauna of Greece, © Antoniadou C et al. -

Coral Reef Algae
Coral Reef Algae Peggy Fong and Valerie J. Paul Abstract Benthic macroalgae, or “seaweeds,” are key mem- 1 Importance of Coral Reef Algae bers of coral reef communities that provide vital ecological functions such as stabilization of reef structure, production Coral reefs are one of the most diverse and productive eco- of tropical sands, nutrient retention and recycling, primary systems on the planet, forming heterogeneous habitats that production, and trophic support. Macroalgae of an astonish- serve as important sources of primary production within ing range of diversity, abundance, and morphological form provide these equally diverse ecological functions. Marine tropical marine environments (Odum and Odum 1955; macroalgae are a functional rather than phylogenetic group Connell 1978). Coral reefs are located along the coastlines of comprised of members from two Kingdoms and at least over 100 countries and provide a variety of ecosystem goods four major Phyla. Structurally, coral reef macroalgae range and services. Reefs serve as a major food source for many from simple chains of prokaryotic cells to upright vine-like developing nations, provide barriers to high wave action that rockweeds with complex internal structures analogous to buffer coastlines and beaches from erosion, and supply an vascular plants. There is abundant evidence that the his- important revenue base for local economies through fishing torical state of coral reef algal communities was dominance and recreational activities (Odgen 1997). by encrusting and turf-forming macroalgae, yet over the Benthic algae are key members of coral reef communities last few decades upright and more fleshy macroalgae have (Fig. 1) that provide vital ecological functions such as stabili- proliferated across all areas and zones of reefs with increas- zation of reef structure, production of tropical sands, nutrient ing frequency and abundance. -

Blooms of the Pelagic Tunicate, <I>Dolioletta Gegenbauri:</I> Are
Journal of Marine Research, 43, 211-236,1985 Blooms of the pelagic tunicate, Dolioletta gegenbauri: Are they associated with Gulf Stream frontal eddies? I 2 by Don Deibel • ABSTRACT Satellite-directed sampling was used to determine whether blooms of Do/ioletta gegenbauri are associated with warm filaments of Gulf Stream frontal eddies. Radio-transmitting drogues were used to mark the center of the bloom so that physical and biological covariables could be measured inside and outside of bloom waters. The bloom was not in the warm filament of a frontal eddy, but was 60 - 70 km northwest of the temperature front between outer-shelf water and the Gulf Stream-in upwelled water probably originating from the eddy's cold core. This cold-core remnant (CCR) water was stranded between 2 middle-shelf fronts. The doliolid bloom resulted from the asexual production of gonozooids by the oozooid stage. This occurred primarily in the nearshore temperature and salinity front and in or beneath the pycnocline between CCR and overriding outer-shelf surface water. Several of the doliolid populations were estimated to be capable of clearing 40-120% of their resident water volume each day-removing particles of less than 50 J.Lm equivalent spherical diameter. Their removal of small particles is thought to be one of the primary reasons for poor copepod recruitment and low net zooplankton concentrations in the midst of doliolid blooms. The phytoplankton community was co-dominated by dinoflagellates and diatoms. indicating the strong influence of the Gulf Stream in these mid-shelf waters. Dominant diatoms were Thalassiosira subti/is and Rhizosolenia sp., both typical of Gulf Stream upwelling in the Georgia Bight.