Evects of Food Availability, Snow and Predation on Breeding Performance of Waders at Zackenberg
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Age-Related Differences in Ruddy Turnstone Foraging and Aggressive Behavior
AGE-RELATED DIFFERENCES IN RUDDY TURNSTONE FORAGING AND AGGRESSIVE BEHAVIOR SARAH GROVES ABSTRACT.--Theforaging behavior of fall migrant Ruddy Turnstoneswas studiedon the Mas- sachusettscoast on 2 different substrates,barnacle-covered rocks and sand and weed-litteredflats. Foragingrates differedsignificantly between the 2 substrates.On eachsubstrate the foragingand successrates of adults and juveniles differed significantly while the frequenciesof successwere similarfor both age-classes.The observeddifferences in foragingrates of adultsand juvenilesmay be due to the degreeof refinementof foragingtechniques. Experience in searchingfor and handling prey may be a primary factor accountingfor thesedifferences, and foragingperformance probably improves with age and experience.Alternatively, the differencesmay be due to the presenceof inefficient juveniles that do not survive to adulthood. Both adultsand juveniles in the tall-depressedposture were dominant in aggressiveinteractions much morefrequently than birds in the tall-levelposture. In mixedflocks of foragingadult and juvenile turnstones,the four possibletypes of aggressiveinteractions occurred nonrandomly. Adult over juvenile interactionsoccurred more frequently than expected,and juvenile over adult interac- tions were never seen.A tentative explanationof this phenomenonmay be that juveniles misinter- pret or respondambivalently to messagesconveyed behaviorally by adultsand thusbecome espe- cially vulnerableto aggressionby adults.The transiencyof migrantsmade it unfeasibleto evaluate -
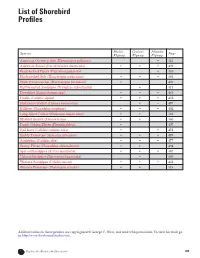
List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

AVIAN PARAMYXOVIRUSES in SHOREBIRDS and Gulls
journal Diseases, 46(2), 2010, pp. 481-487 \Vildlife Disease Association 2010 AVIAN PARAMYXOVIRUSES IN SHOREBIRDS AND GUllS laura l. Coffee,1,5 Britta A. Hanson,' M. Page Luttrell;' David E. Swa~ne,2 Dennis A. Senne,3 Virginia H. Goekjlan," lawrence J. Niles,4,6 and David E. Stallknecht1, 1 Southeastern Cooperative Wildlife Disease Study, Departrnent of Population Health, College of Veterinary Medicine, The University of Georgia, Athens, Georgia 30602, USA 2 Southeast Poultry Research Laboratory, Agricultural Research Service, US Departrnent of Agriculture, Athens, Georgia 30605, USA 3 US Departrnent of Agriculture, Anirnal and Plant Health Inspection Service, National Veterinary Services Laboratories, Ames, Iowa 50010, USA 4 Endangered and Nongame Species Program, New Jersey Division of Fish and Wildlife, PO Box 400, Trenton, New Jersey 08625, USA 5 Current address: Cornell University, College of Veterinary Medicine, S2-118 Schurman Hall, Biomedical Sciences, Ithaca, New York 14853, USA 6 Current address: Conserve Wildlife Foundation, 516 Farnsworth Avenue, Bordertown, New Jersey 08505, USA 7 Corresponding author (email: [email protected]) ABSTRACT: There are nine serotypes of avian paramyxovirus (APMV), including APMV-1, or Newcastle disease virus. Although free-flying ducks and geese have been extensively monitored for APMV, limited information is available for species in the order Charadriiforrnes. From 2000 to 2005 we tested cloacal swabs from 9,128 shorebirds and gulls (33 species, five families) captured in 10 states within the USA and in three countries in the Caribbean and South America. Avian paramyxoviruses were isolated from 60 (0.7%) samples by inoculation of embryonating chicken eggs; isolates only included APMV-1 and APMV-2. -

Bird Observations in Siriuspasset, North Greenland, 2016 and 2017
Bird observations in Siriuspasset, North Greenland, 2016 and 2017 WON YOUNG LEE (Med et dansk resumé: Fugleobservationer i Siriuspasset, Nordgrønland, 2016 og ’17) Abstract During the summer seasons of 2016 and 2017, bird observations were recorded near Siriuspasset in Nansen Land, North Greenland. Breeding birds were surveyed in a 9 km2 area. Wader populations were dense compared to other sites in North and Northeast Greenland, and Red Phalarope Phalaropus fulicarius and Lapland Longspur Calca- rius lapponicus were found to breed far north of their previously known distributions in Greenland. Introduction Description of the study area and methods During the summers of 2016 and 2017, a Danish-Korean The study site was located on the east shore of J. P. Koch expedition performed biological and paleontological Fjord at the southwestern end of Siriuspasset, at alti- studies near Siriuspasset in Nansen Land, North Green- tudes of 0-300 m a.s.l., and here a well-vegetated area land. Compared to the North Greenland region as a of 9 km2 was censused for breeding birds (Figs 1 & 2). whole, Siriuspasset and its surroundings have very lush This was delimited by the coast, a river and features in vegetation, comparable to areas 900 km to the south in the terrain. Northeast Greenland (cf. Fig. 20 in Aastrup et al. 2005). From 25 July to 13 August, 2016 and 30 June to 21 Siriuspasset is a well-known habitat for both muskoxen July, 2017 breeding birds were monitored daily in the Ovibos moschatus and wolves Canis lupus, and high census area. In both years, the survey periods were actu- numbers of moulting Pink-footed Geese Anser brachyr- ally too late to census breeding waders, as failed breed- rhynchus were recorded there in 2009 during an aerial ers may have left by then (cf. -

Arenaria Interpres (Ruddy Turnstone)
Arenaria interpres (Ruddy Turnstone) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 4 Sources of reported national population data p. 7 Species factsheet bibliography p. 10 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Arenaria interpres (Ruddy Turnstone) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

54971 GPNC Shorebirds
A P ocket Guide to Great Plains Shorebirds Third Edition I I I By Suzanne Fellows & Bob Gress Funded by Westar Energy Green Team, The Nature Conservancy, and the Chickadee Checkoff Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 • Identification Tips • 4 Plovers I Black-bellied Plover • 6 I American Golden-Plover • 8 I Snowy Plover • 10 I Semipalmated Plover • 12 I Piping Plover • 14 ©Bob Gress I Killdeer • 16 I Mountain Plover • 18 Stilts & Avocets I Black-necked Stilt • 19 I American Avocet • 20 Hudsonian Godwit Sandpipers I Spotted Sandpiper • 22 ©Bob Gress I Solitary Sandpiper • 24 I Greater Yellowlegs • 26 I Willet • 28 I Lesser Yellowlegs • 30 I Upland Sandpiper • 32 Black-necked Stilt I Whimbrel • 33 Cover Photo: I Long-billed Curlew • 34 Wilson‘s Phalarope I Hudsonian Godwit • 36 ©Bob Gress I Marbled Godwit • 38 I Ruddy Turnstone • 40 I Red Knot • 42 I Sanderling • 44 I Semipalmated Sandpiper • 46 I Western Sandpiper • 47 I Least Sandpiper • 48 I White-rumped Sandpiper • 49 I Baird’s Sandpiper • 50 ©Bob Gress I Pectoral Sandpiper • 51 I Dunlin • 52 I Stilt Sandpiper • 54 I Buff-breasted Sandpiper • 56 I Short-billed Dowitcher • 57 Western Sandpiper I Long-billed Dowitcher • 58 I Wilson’s Snipe • 60 I American Woodcock • 61 I Wilson’s Phalarope • 62 I Red-necked Phalarope • 64 I Red Phalarope • 65 • Rare Great Plains Shorebirds • 66 • Acknowledgements • 67 • Pocket Guides • 68 Supercilium Median crown Stripe eye Ring Nape Lores upper Mandible Postocular Stripe ear coverts Hind Neck Lower Mandible ©Dan Kilby 1 Introduction Shorebirds, such as plovers and sandpipers, are a captivating group of birds primarily adapted to live in open areas such as shorelines, wetlands and grasslands. -

STATUS ASSESSMENT and CONSERVATION RECOMMENDATIONS for the COMMON TERN (Sterna Hirundo) in the GREAT LAKES REGION
STATUS ASSESSMENT AND CONSERVATION RECOMMENDATIONS FOR THE COMMON TERN (Sterna hirundo) IN THE GREAT LAKES REGION Francesca J. Cuthbert Linda R. Wires Kristina Timmerman University of Minnesota Department of Fisheries, Wildlife, & Conservation Biology 1980 Folwell Ave. St. Paul, MN 55108 USA September 2003 For additional copies, contact: Nongame Bird Coordinator U.S. Fish and Wildlife Service Federal Bldg., 1 Federal Drive Fort Snelling, MN 55111-4056 Recommended Citation: Cuthbert, F.J., Wires, L.R. and K. Timmerman. 2003. Status Assessment and Conservation Recommendations for the Common Tern (Sterna hirundo) in the Great Lakes Region. U.S. Department of the Interior, Fish and Wildlife Service, Ft. Snelling, MN. TABLE OF CONTENTS ACKNOWLEDGMENTS………………………………………………………..iv EXECUTIVE SUMMARY…………………………………………………….….1 BACKGROUND……………………………………………………………………..2 INTRODUCTION…………………………………………………….…………………2 TAXONOMY…………………………………………………………………………….3 PHYSICAL DESCRIPTION……………………………………………………………3 RANGE…………………………………………………………………………………...4 BAND RECOVERY DATA AND POPULATION BOUNDARIES……………….…5 HABITAT………………………………………………………………………………...6 Breeding Season Habitat Requirements…………………………………………...6 Post-Breeding Staging Habitat Requirements……………………………………..8 Winter Habitat Requirements……………………………………………………...8 BIOLOGY………………………………………………………………………..………8 Migration and Wintering Grounds………………………………………………...8 Reproduction………………………………………………………………………9 Diet and Foraging Ecology………………………………………………………10 Mortality and Longevity…………………………………………………………11 POPULATION SIZE AND TRENDS………………...………………………..12 -

Shorebird Habitat Conservation Strategy
Upper Mississippi River and Great Lakes Region Joint Venture Shorebird Habitat Conservation Strategy May 2007 1 Shorebird Strategy Committee and Members of the Joint Venture Science Team Bob Gates, Ohio State University, Chair Dave Ewert, The Nature Conservancy Diane Granfors, U.S. Fish and Wildlife Service Bob Russell, U.S. Fish and Wildlife Service Bradly Potter, U.S. Fish and Wildlife Service Mark Shieldcastle, Ohio Department of Natural Resources Greg Soulliere, U.S. Fish and Wildlife Service Cover: Long-billed Dowitcher. Photo by Gary Kramer. i Table of Contents Plan Summary................................................................................................................... 1 Acknowledgements...................................................................................................... 2 Background and Context ................................................................................................. 3 Population Status and Trends ......................................................................................... 6 Habitat Characteristics .................................................................................................. 11 Biological Foundation..................................................................................................... 14 Planning Framework.................................................................................................. 14 Migration and Distribution........................................................................................ 15 Limiting -

Birds of Ohio Shores
Birds of Ohio Shores: Diversity, Ecology and Management of Shorebirds in Ohio Woodlands Stewards Friday Morning Webinar, October 2, 2020 From Plovers to Pipers (who dey): A diversity tour of Ohio Shorebirds 2 Large Plovers: 3 Common Plovers: 4 Uncommon Plovers: 5 Avocet and Black-Necked Stilt 6 Greater and Lesser Yellowlegs 7 Solitary and Spotted Sandpipers 8 Willet and Upland Sandpiper 9 Whimbrel 10 Hudsonian and Marbled Godwits 11 Ruddy Turnstone and Sanderling 12 “Peeps” (= Calidris spp.) Hard to identify; they all look alike and often occur in large flocks. 13 Dublin and Pectoral Sandpiper 14 White-rumped and Baird’s Sandpipers 15 Semi-palmated and Least Sandpipers 16 Stilt and Buff-breasted Sandpipers 17 End of the “peeps” 18 Long and Short-billed Dowitcher 19 American Woodcock and Wilson’s Snipe 20 Wilson’s and Red-necked Phalaropes 21 And if that were not enough! 22 Breeding, juvenile, fall and spring plumages! 23 Prebalternate and prebasic molts (all spp. of shorebirds, not limited to peeps) Feathers wear so plumage changes spring to fall. 24 Shorebird Guilds Body Size Leg Length Bill size and shape Foraging behavior Habitat type (wetland zone) 25 Shorebird Guilds Small gleaners (beach, dry mudflat) Small probers (moist mudflat) Large probers (moist mudflat, shallow water) Large gleaners (shallow water) 26 Shorebird Habitats Meadow/Marsh Deep (er) Water Shallow Water Wet Mudflat Dry Mudflat 27 28 Shorebird Habitats Dry mudflat species: Killdeer Baird’s Sandpiper Buff-breasted Sandpiper Black-bellied Plover Golden Plover 29 -

Wader Population Censuses in the Arctic: Getting the Timing Right HANS MELTOFTE1
ARCTIC VOL. 54, NO. 4 (DECEMBER 2001) P. 367– 376 Wader Population Censuses in the Arctic: Getting the Timing Right HANS MELTOFTE1 (Received 12 May 2000; accepted in revised form 3 January 2001) ABSTRACT. There is increasing evidence that breeding wader censuses often have significantly underestimated densities in the Arctic and other areas. This evidence includes the recording of many more red knots Calidris canutus in the West Palearctic and African wintering areas than can be accounted for on the Arctic breeding grounds. The main causes of underestimated breeding numbers are that many species behave very inconspicuously during incubation, when most censuses have been performed, and that censuses taken later, during the chick-rearing period, record only successful breeding attempts. Records of four seasons at Zackenberg in central Northeast Greenland suggest that in the Arctic, the best population density data are obtained by mapping pairs and territorial individuals during the pair formation, territory establishment, and egg-laying periods, that is, when all birds are present and easiest to record. Revised summer population estimates for Old World wintering Nearctic waders are 20–200% higher than previous estimates. Key words: behaviour, breeding phenology, census methodologies, eastern Canadian Arctic, Northeast Greenland, population densities, population sizes, shorebirds, waders, Zackenberg RÉSUMÉ. On a de plus en plus de preuves que les recensements d’échassiers nicheurs ont souvent largement sous-estimé les densités dans l’Arctique et dans d’autres régions. Ces preuves comprennent le fait que l’on recense beaucoup plus de bécasseaux maubèches Calidris canatus dans les aires d’hivernage du paléarctique occidental et d’Afrique que l’on ne peut en compter sur les lieux de reproduction dans l’Arctique. -
![Ruddy Turnstone Arenaria Interpres [M,W]](https://docslib.b-cdn.net/cover/1956/ruddy-turnstone-arenaria-interpres-m-w-3381956.webp)
Ruddy Turnstone Arenaria Interpres [M,W]
Appendix A: Birds Ruddy Turnstone Arenaria interpres [M,W] Federal Listing N/A State Listing N/A Global Rank G5 State Rank SNR Regional Status Very High Photo by Pamela Hunt Justification (Reason for Concern in NH) Populations of several migratory shorebirds are in steep decline (Andres 2009, Winn et al. 2013). Based largely on these declines, several species were proposed as RSGCN for the Northeast, and those that occur regularly in NH are included in the 2015 NH Wildlife Action Plan. Distribution Ruddy Turnstones breed across the arctic from Alaska to Siberia, and have an extensive wintering range along south temperate and tropical coastlines worldwide (Nettleship 2000). In New Hampshire the species is relatively uncommon, and limited to the immediate coast and Isles of Shoals. Most occur during migration in August and September, with lesser numbers in May. There are also a few winter records, but the species is quite rare between November and April. Habitat During the non‐breeding season, turnstones forage primarily on rocky shorelines, although they will occasionally use beaches and mudflats. NH Wildlife Action Plan Habitats ● Coastal Islands ● Estuarine ● Dunes Distribution Map New Hampshire Wildlife Action Plan Appendix A Birds-50 Appendix A: Birds Current Species and Habitat Condition in New Hampshire Populations of many long‐distance migrant shorebirds are believed to be in steep decline, and for this reason several species are considered priorities for future conservation. Although data for Ruddy Turnstone is somewhat equivocal, most recent assessments suggest its populations in North America are stable or increasing (Morrison et al. 1994, Morrison et al.