Lab-Tests-Code.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood, Lymph, and Immunity Joann Colville
Blood, Lymph, and Immunity Joann Colville CHAPTER OUTLINE .»: BLOOD Function Introduction Lymphatic Structures Plasma THE IMMUNE SYSTEM Cellular Components of Blood Function Red Blood Cells Immune Reactions Platelets Immunization: Protection Against Disease White Blood Cells THE LYMPHATICSYSTEM Lymph Formation Characteristics LEARNING OBJECTIVES • List and describe the functions of blood • List the functions of the lymphatic system • Describe the composition of blood plasma • Describe the structure and functions of the lymph nodes, • Describe the characteristics of mature erythrocytes spleen, thymus, tonsils, and GALT Describe the structure of the hemoglobin molecule and • List the functions of the immune system explain the fate of hemoglobin following intravascular and • Differentiate between specific and nonspecific immune extravascular hemolysis reactions • Give the origin of thrombocytes and describe their • Differentiate between cell-mediated and humoral immunity characteristics and functions • List the components involved in cell-mediated immunity • Listthe types of leukocytes and describe the functions of each and explain the role of each • Describe the formation of lymph fluid and its circulation List and describe the classes of immunoglobulins through the lymphatic system • Differentiate between active and passive immunity BLOOD vessels of the cardiovascular system. It has three main func- tions: transportation, regulation, and defense. INTRODUCTION Blood. Say the word, and some people cringe, others faint, Function and if you believe authors Bram Stoker, Stephen King, and 1. Blood is a transport system. Christopher Moore (all authors of vampire books), others • It carries oxygen, nutrients, and other essential com- drool. But no matter what you think about blood, animals pounds to every living cell in the body. -

Evaluation of Abnormal Liver Chemistries
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD1, Stanley M. Cohen, MD, FACG, FAASLD2, and Joseph K. Lim, MD, FACG, FAASLD3 1Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California, USA; 2Digestive Health Institute, University Hospitals Cleveland Medical Center and Division of Gastroenterology and Liver Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA; 3Yale Viral Hepatitis Program, Yale University School of Medicine, New Haven, Connecticut, USA. Am J Gastroenterol 2017; 112:18–35; doi:10.1038/ajg.2016.517; published online 20 December 2016 Abstract Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defined as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defined as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. -

Total Bilirubin in Athletes, Determination of Reference Range
OriginalTotal bilirubin Paper in athletes DOI: 10.5114/biolsport.2017.63732 Biol. Sport 2017;34:45-48 Total bilirubin in athletes, determination of reference range AUTHORS: Witek K1, Ścisłowska J2, Turowski D1, Lerczak K1, Lewandowska-Pachecka S2, Corresponding author: Pokrywka A3 Konrad Witek Department of Biochemistry, Institute of Sport - National 1 Department of Biochemistry, Institute of Sport - National Research Institute, Warsaw, Poland Research Institute 2 01-982 Warsaw, Poland Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Poland 3 E-mail: Department of Biochemistry, 2nd Faculty of Medicine, Medical University of Warsaw, Poland [email protected] ABSTRACT: The purpose of this study was to determine a typical reference range for the population of athletes. Results of blood tests of 339 athletes (82 women and 257 men, aged 18-37 years) were retrospectively analysed. The subjects were representatives of different sports disciplines. The measurements of total bilirubin (BIT), iron (Fe), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were made using a Pentra 400 biochemical analyser (Horiba, France). Red blood cell count (RBC), reticulocyte count and haemoglobin concentration measurements were made using an Advia 120 haematology analyser (Siemens, Germany). In groups of women and men the percentage of elevated results were similar at 18%. Most results of total bilirubin in both sexes were in the range 7-14 μmol ∙ L-1 (49% of women and 42% of men). The highest results of elevated levels of BIT were in the range 21-28 μmol ∙ L-1 (12% of women and 11% of men). There was a significant correlation between serum iron and BIT concentration in female and male athletes whose serum total bilirubin concentration does not exceed the upper limit of the reference range. -

The Diagnosis of Paroxysmal Nocturnal Hemoglobinuria: Role of Flow Cytometry
THE DIAGNOSIS OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA: ROLE OF FLOW CYTOMETRY Alberto Orfao Department of Medicine, Cancer Research Centre (IBMCC-CSIC/USAL), University of Salamanca, Salamanca, Spain Paroxysmal nocturnal hemoglobinuria (PNH) on one or multiple populations of peripheral blood is an acquired clonal disorder typically affecting red cells and/or leucocytes of PNH patients, this young adults, which involves the phosphatidylino- translating into a new diagnostic test. Because sitol glycan anchor biosynthesis class A gene of this flow cytometry became an essential tool (PIGA) coded in chromosome X, in virtually every in the diagnosis of PNH. In turn, it could be also case. The altered gene encodes for a defective pro- demonstrated that investigation of the presence of tein product, the pig-a enzyme which is involved GPI-deficient cells among circulating mature neu- in the early steps of the synthesis of glycosylphos- trophils and monocytes, increases the sensitivity phatidylinositol (GPI). This later molecule (GPI) of red blood cell screening because of the shorter acts as an anchor of a wide range of proteins to lifetime of both populations of leucocytes. Despite the cell surface. At present a large number of GPI- all the above, routine assessment of CD55 and anchor associated proteins have been described CD59 is also associated with several limitations which are expressed by one or multiple distinct due to distinct patterns of expression among dif- compartments of hematopoietic cells. The altered ferent cell populations, dim and heterogeneous PIGA gene leads to an altered GPI anchor which expression among normal individuals and the lack leads to defective expression of GPI-AP on the of experience in many labs as regards the normal cytoplasmic membrane, on the cell surface. -

Normal Rangesа–А
Effective August 2007 5361 NW 33 rd Avenue Fort Lauderdale, FL 33309 954-777-0018 NORMAL RANGES – fax: 954-777-0211 ADULT MALE FEMALE CBC WBC 3.6 – 11.0 K/UL 3.6 – 11.0 RBC 4.5 – 5.9 M/UL 4.5 – 5.9 HEMOGLOBIN 13.0 – 18.0 G/DL 12.0 – 15.6 HEMATOCRIT 40 – 52 % 36.0 – 46.0 MCV 81 – 97 FL 81 93 MCH 26 – 34 PG 26 34 MCHC 31 – 37 gm/dl 31 37 RDW 11.5 – 15 % 11.5 – 15.0 PLATELET CT 150 – 400 K/UL 140 400 PLT VOL 7.4 – 10.4 FL 7.4 – 10.4 NEUTROPHIL % 36 – 66 36 75 LYMPHOCYTE % 23 – 43 27 47 MONOCYTE % 0.0 – 10 0.0 10 EOSINOPHIL % 0 – 5.0 0.0 – 5.0 BASOPHIL % 0 – 1.0 0.0 – 1.0 RETIC 0.4 – 1.9 0.4 – 1.9 COMPREHENSIVE METABOLIC PROFILE /LIVER PROFILE SODIUM 135 – 145 MMOL/L 135 145 POTASSIUM 3.5 – 5.5 MMOL/L 3.5 – 5.5 (OUTREACH) CHLORIDE 95 – 110 MMOL/L 95 110 CO2 19 – 34.MMOL/L 19 34 GLUCOSE 70 – 110 MG/DL 70 110 BUN 6 – 22 MG/DL 6 22 CREATININE 0.6 – 1.3 MG/DL 0.6 – 1.3 MG/DL CALCIUM 8.4 – 10.2 MG/DL 8.4 – 10.2 TOTAL PROTEIN 5.5 – 8.7 G/DL 5.5 – 8.7 ALBUMIN 3.2 – 5.0 g/dl 3.2 – 5.0 BILI TOTAL 0.1 – 1.2 MG/DL 0.1 – 1.2 BILI DIRECT 0.0 – 0.3 MG/DL 0.0 – 0.3 BILI INDIRECT 0.2 – 1.0 MG/DL 0.2 – 1.0 ALKALINE PHOS 20 – 130 U/L 20 130 AST/SGOT 10 – 40 U/L 10 40 ALT/SGPT 7 55 U/L 7 55 AST/ALT RATIO 0.5 – 0.9 RATIO 0.5 – 0.9 AMYLASE 25 – 115 U/L 25 – 115 U/L LIPASE 114 – 286 U/L 114 – 286 U/L CRP 0 – 0.9 MG/DL 0 – 0.9 MG/DL PREALBUMIN 18.0 – 35.7 mg/dL 18.0 – 35.7 mg/dL Revised 807 MAGNESIUM 1.8 – 2.4 mg/dL 1.8 – 2.4 mg/Dl LDH 100 – 200 Units /L 100 – 200 Units/L PHOSPHOROUS 2.3 – 5.0 mg/dL 2.3 – 5.0 mg/dL LACTIC ACID 0.4 -

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -
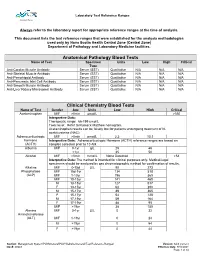
Laboratory Test Reference Ranges
Laboratory Test Reference Ranges Central Zone Always refer to the laboratory report for appropriate reference ranges at the time of analysis. This document lists the test reference ranges that were established for the analysis methodologies used only by Nova Scotia Health Central Zone (Central Zone) Department of Pathology and Laboratory Medicine facilities. Anatomical Pathology Blood Tests Name of Test Specimen Units Low High Critical Type Anti-Cardiac Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Skeletal Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pemphigoid Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pancreatic Islet Cell Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Smooth Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Liver Kidney Microsomal Antibody Serum (SST) Qualitative N/A N/A N/A Clinical Chemistry Blood Tests Name of Test Gender Age Units Low High Critical Acetaminophen M/F >0min µmol/L >350 Interpretive Data: Therapeutic range: 66-199 umol/L Toxic level: Refer to Rumack Matthew nomogram. Acetaminophen results can be falsely low for patients undergoing treatment of N- acetylcysteine (NAC). Adrenocorticotropic M/F >0min pmol/L 2.3 10.1 hormone Interpretive Data: Adrenocorticotropic Hormone (ACTH) reference ranges are based on (ACTH) samples collected prior to 10 AM. Albumin M/F 0-1yr g/L 25 46 >1yr 35 50 Alcohol M/F >0min mmol/L None Detected >54 Interpretive Data: The method is intended for clinical purposes only. Medical-legal specimens should be analyzed by gas chromatographic method for confirmation of results. -

Leukocyte Esterase Activity in Vaginal Fluid of Pregnant and Non-Pregnant Women with Vaginitis/Vaginosis and in Controls
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Infect Dis Obstet Gynecol 2003;11:19–26 Leukocyte esterase activity in vaginal fluid of pregnant and non-pregnant women with vaginitis/vaginosis and in controls Per-Anders Mårdh 1, Natalia Novikova 1, Ola Niklasson 1, Zoltan Bekassy 1 and Lennart Skude 2 1Department of Obstetrics and Gynecology, University Hospital, Lund and 2Department of Clinical Chemistry, County Hospital, Halmstad, Sweden Objectives: Todeterminethe leukocyteesterase (LE) activity invaginal lavage fluid ofwomen with acuteand recurrentvulvovaginal candidosis (VVC and RVVC respectively), bacterial vaginosis(BV), and in pregnant and non-pregnantwomen without evidenceof the threeconditions. Also tocompare the resultof LEtestsin women consultingat differentweeks inthe cycle andtrimesters of pregnancy.The LEactivity was correlatedto vaginal pH, numberof inflammatory cells instained vaginal smears,type of predominatingvaginal bacteria andpresence of yeast morphotypes. Methods: Onehundred and thirteen women with ahistoryof RVVC,i.e. with at least fourattacks of the conditionduring the previousyear and who had consultedwith anassumed new attack ofthe condition,were studied.Furthermore, we studied16 women with VVC,15 women with BV,and 27 women attendingfor control of cytological abnormalities, who all presentedwithout evidenceof either vaginitisor vaginosis. Finally, 73 pregnantwomen wereinvestigated. The LEactivity invaginal fluid duringdifferent weeks inthe cycle of 53 of the women was measured. Results: Inthe non-pregnantwomen, anincreased LE activity was foundin 96, 88, 73 and56% of those with RVVC,VVC and BV andin the non-VVC/BVcases,respectively. In 73% of pregnantwomen inthe second trimester,and 76% of thosein the third,the LEtestwas positive.In all groupsof non-pregnantwomen tested, the LEactivity correlatedwith the numberof leukocytesin vaginal smears,but it didnot in those who were pregnant.There was nocorrelation between LE activity andweek incycle. -

A Rapid and Quantitative D-Dimer Assay in Whole Blood and Plasma on the Point-Of-Care PATHFAST Analyzer ARTICLE in PRESS
MODEL 6 TR-03106; No of Pages 7 ARTICLE IN PRESS Thrombosis Research (2007) xx, xxx–xxx intl.elsevierhealth.com/journals/thre REGULAR ARTICLE A rapid and quantitative D-Dimer assay in whole blood and plasma on the point-of-care PATHFAST analyzer Teruko Fukuda a,⁎, Hidetoshi Kasai b, Takeo Kusano b, Chisato Shimazu b, Kazuo Kawasugi c, Yukihisa Miyazawa b a Department of Clinical Laboratory Medicine, Teikyo University School of Medical Technology, 2-11-1 Kaga, Itabashi, Tokyo 173-8605, Japan b Department of Central Clinical Laboratory, Teikyo University Hospital, Japan c Department of Internal Medicine, Teikyo University School of Medicine, Japan Received 11 July 2006; received in revised form 7 December 2006; accepted 28 December 2006 KEYWORDS Abstract The objective of this study was to evaluate the accuracy indices of the new D-Dimer; rapid and quantitative PATHFAST D-Dimer assay in patients with clinically suspected Deep-vein thrombosis; deep-vein thrombosis (DVT). Eighty two consecutive patients (34% DVT, 66% non-DVT) Point-of-care testing; with suspected DVT of a lower limb were tested with the D-Dimer assay with a Chemiluminescent PATHFAST analyzer. The diagnostic value of the PATHFAST D-Dimer assay (which is immunoassay based on the principle of a chemiluminescent enzyme immunoassay) for DVT was evaluated with pre-test clinical probability, compression ultrasonography (CUS). Furthermore, each patient underwent contrast venography and computed tomogra- phy, if necessary. The sensitivity and specificity of the D-Dimer assay using 0.570 μg/ mL FEU as a clinical cut-off value was found to be 100% and 63.2%, respectively, for the diagnosis of DVT, with a positive predictive value (PPV) and negative predictive value (NPV) of 66.7% and 100%, respectively. -

Determination of Reference Ranges for Selected Clinical Laboratory Tests for a Medical Laboratory in Namibia Using Pre-Tested Data
DETERMINATION OF REFERENCE RANGES FOR SELECTED CLINICAL LABORATORY TESTS FOR A MEDICAL LABORATORY IN NAMIBIA USING PRE-TESTED DATA by CORNELIA DE WAAL-MILLER Student no: 197084516 Thesis submitted in fulfillment of the requirements of the degree Master of Technology: Biomedical Technology in the Faculty of Health and Wellness Sciences at the Cape Peninsula University of Technology Supervisor: Professor A.J. Esterhuyse Co-Supervisor: Professor B. Noden Bellville March 2015 CPUT copyright information The thesis may not be published either in part (in scholarly, scientific or technical journals), or as a whole (as a monograph), unless permission has been obtained from the University. (i) Declaration I, Cornelia de Waal-Miller, declare that the content of this thesis represents my own unaided work, and that this thesis has not previously been submitted for academic examination towards any qualification. Furthermore, this thesis represents my own opinions and not necessarily those of the Cape Peninsula University of Technology. 12th March 2015 Signed Date 2 | of 84 Pages (ii) Abstract Aim: The aim of the study was to compile pre-tested laboratory results stored in the laboratory database of the Namibia Institute of Pathology (NIP). The study also aimed to assess the usefulness and validity of using retrospective laboratory results of different patients in varying degrees of health and which were produced using various methods in different laboratories in Namibia. Methods: 254,271 test results (female: 134,261, male = 117,091, unknown gender= 2,919) consisting of Haemoglobin, serum Urea, serum Creatinine, plasma Glucose (fasting and random), serum Cholesterol, serum Triglycerides and serum Uric Acid was extracted from NIP Laboratory Information System over a period of four years and of the 13 different regions of Namibia were analyzed. -

Chapter 06 Lecture Outline
Chapter 06 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © 2016 McGraw-Hill Education. Permission required for reproduction or display. 1 Cardiovascular System: Blood © SPL/Science Source, (inset) © Andrew Syred/Science Source 2 Points to ponder • What type of tissue is blood and what are its components? • What is found in plasma? • Name the three formed elements in blood and their functions. • How does the structure of red blood cells relate to their function? • Describe the structure and function of each white blood cell. • What are disorders of red blood cells, white blood cells, and platelets? • What do you need to know before donating blood? • What are antigens and antibodies? • How are ABO blood types determined? • What blood types are compatible for blood transfusions? • What is the Rh factor and how is this important to pregnancy? • How does the cardiovascular system interact with other systems to maintain homeostasis? 3 6.1 Blood: An Overview What are the functions of blood? • Transportation: oxygen, nutrients, wastes, carbon dioxide, and hormones • Defense: against invasion by pathogens • Regulatory functions: body temperature, water- salt balance, and body pH 4 6.1 Blood: An Overview What is the composition of blood? • Remember: blood is a fluid connective tissue. • Formed elements are produced in red bone marrow. – Red blood cells/erythrocytes (RBCs) – White blood cells/leukocytes (WBCs) – Platelets/thrombocytes 5 6.1 Blood: An Overview What is the composition of blood? • Plasma – It consists of 91% water and 9% salts (ions) and organic molecules. – Plasma proteins are the most abundant organic molecules. -

Ileal Neobladder: an Important Cause of Non-Anion Gap Metabolic Acidosis
The Journal of Emergency Medicine, Vol. 52, No. 5, pp. e179–e182, 2017 Ó 2017 Elsevier Inc. All rights reserved. 0736-4679/$ - see front matter http://dx.doi.org/10.1016/j.jemermed.2016.12.036 Clinical Communications: Adult ILEAL NEOBLADDER: AN IMPORTANT CAUSE OF NON-ANION GAP METABOLIC ACIDOSIS Jesse W. St. Clair, MD and Matthew L. Wong, MD, MPH Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts and Department of Emergency Medicine, Harvard Medical School, Boston, Massachusetts Reprint Address: Matthew L. Wong, MD, MPH, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Rosenberg Building, 2nd Floor, 1 Deaconess Road, Boston, MA 02446 , Abstract—Background: The differential diagnosis for a disease, or medications such as acetazolamide. non-anion gap metabolic acidosis is probably less well Commonly used mnemonics to remember the differential known than the differential diagnosis for an anion gap for the non-anion gap metabolic acidosis include metabolic acidosis. One etiology of a non-anion gap acidosis ‘‘ABCD’’ for ‘‘Addison’s, Bicarbonate loss, Chloride is the consequence of ileal neobladder urinary diversion (administration), and Drugs,’’ as well as ‘‘HARDUP’’ for the treatment of bladder cancer. Case Report: We pre- for ‘‘Hyperchloremia, Acetazolamide and Addison’s, sent a case of a patient with an ileal neobladder with a severe non-anion gap metabolic acidosis caused by a Renal Tubular Acidosis, Diarrhea, Ureteroenterostomies, urinary tract infection and ureteroenterostomy. Why and Pancreaticoenterostomies’’ (Table 1) (1). Should an Emergency Physician Be Aware of This?: Part Ureteroenterostomies are when the ureter has an of the ileal neobladder surgery includes ureteroenteros- abnormal connection with a segment of intestine.