Analysis of Fruit Juices and Drinks of Ascorbic Acid Content
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food and Drug Administration, HHS § 146.185
Food and Drug Administration, HHS § 146.185 of the food, without intervening writ- weight of the preservative used. If the ten, printed, or graphic matter. food is packed in container sizes that are less than 19 liters (5 gallons), the [42 FR 14414, Mar. 15, 1977, as amended at 44 FR 36378, June 22, 1979; 58 FR 2881, Jan. 6, label shall bear a statement indicating 1993] that the food is for further manufac- turing use only. § 146.153 Concentrated orange juice (e) Wherever the name of the food ap- for manufacturing. pears on the label so conspicuously as (a) Concentrated orange juice for to be easily seen under customary con- manufacturing is the food that com- ditions of purchase, the statement plies with the requirements of com- specified in paragraph (d) of this sec- position and label declaration of ingre- tion for naming the preservative ingre- dients prescribed for frozen con- dient used shall immediately and con- centrated orange juice by § 146.146, ex- spicuously precede or follow the name cept that it is either not frozen or is of the food, without intervening writ- less concentrated, or both, and the or- ten, printed, or graphic matter. anges from which the juice is obtained [42 FR 14414, Mar. 15, 1977, as amended at 44 may deviate from the standards for FR 36378, June 22, 1979; 58 FR 2882, Jan. 6, maturity in that they are below the 1993] minimum Brix and Brix-acid ratio for such oranges: Provided, however, that § 146.185 Pineapple juice. the concentration of orange juice solu- (a) Identity. -

How to Fight Citrus Greening Disease (And It’S Not Through Genetic Engineering)
William & Mary Environmental Law and Policy Review Volume 40 (2015-2016) Issue 3 Article 7 May 2016 Saving The Orange: How to Fight Citrus Greening Disease (And It’s Not Through Genetic Engineering) Evan Feely Follow this and additional works at: https://scholarship.law.wm.edu/wmelpr Part of the Agriculture Law Commons, and the Environmental Law Commons Repository Citation Evan Feely, Saving The Orange: How to Fight Citrus Greening Disease (And It’s Not Through Genetic Engineering), 40 Wm. & Mary Envtl. L. & Pol'y Rev. 893 (2016), https://scholarship.law.wm.edu/wmelpr/vol40/iss3/7 Copyright c 2016 by the authors. This article is brought to you by the William & Mary Law School Scholarship Repository. https://scholarship.law.wm.edu/wmelpr SAVING THE ORANGE: HOW TO FIGHT CITRUS GREENING DISEASE (AND IT’S NOT THROUGH GENETIC ENGINEERING) EVAN FEELY* INTRODUCTION The orange is dying. With Florida’s citrus industry already suffer- ing from the growing skepticism of an increasingly health-conscious American public as to orange juice’s benefits,1 the emergence of citrus greening disease over the past two decades has left the orange’s long-term future very much in doubt.2 A devastating virus first documented in China roughly one hundred years ago, citrus greening disease (or “HLB”), has only migrated to Florida in the past twenty years, but has quickly made up for lost time.3 Primarily transmitted by an insect known as the Asian citrus psyllid (“ACP”), the disease has devastated Florida growers in recent years, wiping out entire groves and significantly affecting trees’ overall yield.4 This past year, Florida growers experienced their least productive harvest in forty years, and current estimates of next year’s yield are equally dismal.5 * J.D. -

THICK & EASY Clear Thickened Prune Drink
Item Number: 72461 Product THICK & EASY Clear Name: Thickened Prune Drink - Nectar - Lvl 2 - 24/4oz Master Item Name: BEV-THKRTS 24/4 PRNE-NCT L2 Product Fact Sheet Product Information UDEX Information UCC Manufacturer ID: 99429 UDEX Department: 17 - NON-ALCOHOLIC BEVERAGES Manufacturer Sequence: 284 UDEX Category: 144 - NON-ALCOHOLIC BEVERAGES OTHER - READY TO DRINK UDEX Sub Category: 484 - JUICE/NECTAR DRINKS - READY TO DRINK Brand: GPC Code: 10000222 - Juice Drinks - Ready to Drink (Perishable) Specifications Ship Container UPC: 10099429724618 Storage Range Unit UPC: Recommended: 70 F Specification Number: 10207 Maximum: 90 F Pallet Code: 12 Minimum: 40 F Pallet Pattern: 10 x 17 = 170 Description: Do Not Freeze Full Pallet: 1288.60 lbs. Catch Weight? NO Kosher? Yes Leaker Allowance: N Contains Allergens: No Allergens present Truckload Quantity: 33 Bioengineering Information: Has not been evaluated for BE content. Total Code Days: 365 Min Delivered Shelf Life Days: Master Dimensions Case Dimensions: 15.94''L x 10.63''W x 2.69''H Cubic Feet: .260 CUFT Unit Quantity: 24 Net Weight: 6.82 LB Unit Size: Gross Weight: 7.58 LB Pack: CASE Tare Weight: .76 LB Nutrition Facts Domestic Nutrition Only Method: Product Form: NLEA Adjusted Values: Y Label Number: Child Nutrition Label: Food Category Code: Recipe Code: Source Code: Product Description General Description: THICK & EASY Clear Thickened Prune Drink - Nectar - Lvl 2 - 24/4oz Benefits of Using This Product: Pre-thickened prune juice is a great tasting alternative to other juices with laxative benefits as well. Product Claims: KOSHER - CIRCLE U - ORTHODOX UNION Nutrition Claims: Excellent Source of Fiber List of Ingredients: Nectar Consistency, 10 % Juice from Concentrate with Added Ingredients Ingredients: Water, Sugar, Prune Base (Concentrated Prune Juice, Caramel Color, Water, Natural Flavor, Citric Acid, Gum Arabic, Sunflower Oil, Ascorbic Acid [Vitamin C], Glycerol Ester of Wood Rosin, Potassium Sorbate [Preservative], Brominated Vegetable Oil), Resistant Maltodextrin, Xanthan Gum, Ascorbic Acid, Citric Acid. -

Cuisinart® Juice Extractor CJE-1000
INSTRUCTION BOOKLET Cuisinart® Juice Extractor CJE-1000 For your safety and continued enjoyment of this product, always read the instruction book carefully before using. 16. The appliance is wired for domestic use only. IMPORTANT 17. Do not use the appliance for anything other than the intended purpose, as outlined SAFEGUARDS in the instruction booklet. When using any electrical appliance, basic safety precautions should always be followed, including 18. Never juice with the spout in the closed position. the following: 19. Do not operate without the pulp container 1. READ INSTRUCTIONS THOROUGHLY in place. 2. Always unplug unit from outlet when not in 20. Do not operate your appliance in an appliance use, before putting on or removing parts garage or under a wall cabinet. When storing in and before cleaning. an appliance garage always unplug the unit from the electrical outlet. Not doing so could 3. To protect against electrical shock, do not create a risk of fire, especially if the appliance immerse the juice extractor motor housing in touches the walls of the garage or the door water or other liquids. touches the unit as it closes. 4. When any appliance is used by or near children, supervise closely. 5. Avoid contact with moving parts. SAVE THESE 6. If the juice extractor has a damaged cord or INSTRUCTIONS plug or malfunctions, DO NOT OPERATE. Contact our Customer Service Center to return for examination, repair or adjustment. HOUSEHOLD USE ONLY 7. Using accessory attachments not sold or recommended by the manufacturer can cause No user-serviceable parts are inside. Do not fire, electric shock or injury. -

Holiday Cheer Mocktails
HolidayHoliday CheerCheer MocktailsMocktails This holiday season, please remember... Be safe and smart - Don't Drink and Drive! When you're toasting the season, why not have one... without? Toast the season with your friends & family using one of these recipes for delicious, alcohol-free mixed drinks. Fruit Fizz Candy Cane Punch Cardinal Punch 1/3 cup pineapple juice ¼ cup lemon juice 1 quart cranberry juice 1/3 cup orange juice 16-ounce can orange juice 1 pint orange juice 1/3 cup lime juice concentrate 2 lemons squeezed or 2 ouncesounce 1 teaspoon powdered sugar ¼ cup sugar lemon juice club soda 1 egg white 4 quarts ginger ale grated lemon rind or splash lemon 6 hard peppermint candies Combine juice with ice and chill. juice 4 peppermint candy canes Pour into punch bowl overer a blockbl Add juice to a shaker of cracked ice. Ginger ale of ice and add ginger ale. Garnishrnish Shake, strain into cocktail glass or Put all ingredients except candy glasses with mint sprig. May adddd tumbler, top with club soda. Garnish canes and ginger ale in blender. orange sherbet scoops. Serves 10. with lemon or lime twist. Blend until candies liquefy. Divide evenly between 4 tall glasses and top with ginger ale. Stir gently to mix. Mock Pink Champagne OJ Sparkle Serve with peppermint candy canes ½ cup sugar 2 ounces orange juice as stirrers. (serves 4) 1 cup water 3 ounces lemon-lime soda 1 (6( ounce) can frozen orange juice 1 ounce maraschino cherry juiceice concentrate Blend ingredients and serve overr ice Sparkling Fruit Slush 1 (6 ounce) can frozen grapefruit in large glass. -

Squeezed Orange Juice Properties Before and During <I>In Vitro</I>
Fresh-Squeezed Orange Juice Properties Before and During In Vitro Digestion as Influenced by Orange Variety and Processing Method Yamile A. Mennah-Govela and Gail M. Bornhorst Abstract: This study aims to analyze the influence of processing and orange variety on initial quality, antioxidant activity, total polyphenol content (TPC), and ascorbic acid content of fresh-squeezed orange juice during in vitro digestion. Fresh- squeezed orange juice was made from Fukumoto Navel, Lane Late Navel, Olinda Nucellar Valencia, and Campbell Valencia oranges, and was processed thermally and nonthermally. Antioxidant activity (FRAP and ABTS assays), TPC (Folin-Ciocalteu method), and ascorbic acid were analyzed before and after gastrointestinal digestion. Bioaccessibility was calculated by comparing the measured values after digestion with the initial value for each juice. Orange variety significantly influenced pH, acidity, and total soluble solids (P < 0.0001). Antioxidant activity by FRAP was significantly higher (P < 0.0001) in Fukumoto Navel orange juice (16.0 ± 0.4 mM Trolox) than the other juices (range: 9.1 to 10 mM Trolox). TPC was significantly influenced by orange variety (P < 0.0001) and ranged from 521 ± 6 (Campbell Valencia) to 800 ± 11 mg gallic acid/L (Lane Late Navel). Processing method did not influence antioxidant and polyphenol bioaccessibility (P > 0.05). However, antioxidant activity by ABTS and ascorbic acid bioaccessibility were significantly influenced by orange variety (P < 0.0001). These results indicate that fruit variety and nutrient bioaccessibility should be considered to optimize processing and formulation parameters. Keywords: antioxidant activity, ascorbic acid, bioaccessibility, in vitro digestion, orange juice Practical Application: Processing method and variety of fruit during beverage manufacturing may influence its nutrient bioaccessibility. -

Grapes and Lemons Are Two of Nature's Greatest Lymphatic Cleansers And
GrapeGate v1.0 [email protected] Written by: Robert Morse, N.D. “Grapes and lemons are two of nature’s greatest lymphatic cleansers and ‘tumor busters.’ I have seen lymphomas gone in forty-five days and stomach cancer gone in fifty-nine days using these fruit juice fasts in combination with herbal therapy and a raw food diet.” A word of caution (Nichlas Brandon): Bear in mind that a lemonade or grape juice fast are both powerful approaches to initiating the cleansing process. Those whom are just starting out on their new journey toward regaining health from a past of defiling their bodies with overly-processed junk foods, dairy products, sodas, caffeine, smoking, and so forth, should proceed cautiously and consider that moving straight into a fast of this magnitude - too soon - can throw a person into rapid and strong cycles of elimination which are NOT tolerated or handled well by many. Therefore, unless circumstances are urgent and immediate [fruit] juice fasting is called for, it is wise that you take your time, begin your transition and work yourself up in preparation for whichever fast you decide to undergo. “Dipping your toe in the water” with a mild green juice fast (≤2 US quarts of juice daily) or feast (>2 US quarts of juice daily) will give you a first impression of what’s in store for you with the more aggressive fruit juice fasts - and what it’s like to abstain from solid food for longer periods of time. Having some degree of good intestinal health is also important prior to commencing a fast, hence, in preparation for the fast, spending at least 1-3 months on a diet rich in raw fruits and vegetables along with a restorative herbal intestinal formula (e.g. -

Cocktail Menu
ARNAUDVILLE LOUISIANA COCKTAILS SIGNATURES SIGNATURES OLD FASHIONED 10 AVIATION 11 bourbon, orange slice, duo of bitters simple syrup, gin, luxardo maraschino, lemon juice, creme de violette cherry BAYOU PLANTERS PUNCH 10 BUTTER PECAN OLD FASHIONED 10 silver rum, bayou satsuma rum, orange, pineapple & lime bourbon, butter pecan syrup, orange slice, duo of juice, grenadine bitters, cherry 5TH AVE 10 THE LITTLE BIG HURRICANE 10 makers mark, orange juice, champagne, lemon juice bayou satsuma rum, passion fruit rum, mango nectar, watermelon puree, orange juice, lime juice, pineapple NIGHT OF THE LIVING PINEAPPLE 10 tequila, pineapple juice, disaronno amaretto juice SANJAY'S MANHATTAN 10 BEES KNEES 9 bulleit rye, nolly prat rouge, bitters, cherry gin, lemon juice, caramelized honey syrup DARK N STORMY 10 JUNGLE BIRD 10 silver rum, blackstrap rum, lime juice, ginger beer blackstrap rum, campari, pineapple juice, lime, simple syrup FRENCH MARTINI 10 vodka, chambord, pineapple juice TIGERS PRIDE 10 vodka, blueberry lemonade, fresh blueberries, gold ARNAUDVILLE COSMO 10 sugar vodka, pineapple, lime & cranberry juice, triple sec LA LOUISIANE 10 PEAR, ELDER FLOWER MARTINI 10 rye, sweet vermouth, green chartreuse, bitters, pear vodka, elderflower, lemon juice absinthe BRUNCH FRENCH 75 8 champagne, gin, lemon juice MIMOSA 6 orange juice, champagne FRENCH 77 9 champagne, gin, lemon juice, elderflower THE LITTLE BIG MARY 8 vodka, house bloody mary mix, pickled veggies BRUNCH PUNCH 8 champagne, vodka, orange juice, pineapple juice, mint, FLAVORED MIMOSA 7 blueberry, mango, grapefruit, watermelon, pineapple, berries cranberry, blood orange HOUSE PIMMs CUP 9 MIMOSAS FOR THE TABLE 35 pimms no 1, lemon juice, ginger ale, cucumber HONEY BEE 8 rum, honey, lemon juice we strongly advise caution when drinking & walking down SUNSHINE 8 to the bayou. -

Household Juice Extractor Comparison and Optimization
cess Pro ing d & o o T F e c f h o n l o a l n o Journal of Food Processing and r g u y o J ISSN: 2157-7110 Technology Research Article Household Juice Extractor Comparison and Optimization Michael Donaldson* Hallelujah Acres, Zillah, WA, USA ABSTRACT Background: Increased vegetable consumption is a cornerstone of improving personal dietary habits and meeting national dietary guidelines. Vegetable juice is a beneficial way to increase vegetable consumption. Aim: The aim was to scientifically compare six different juicers for quantity and quality of juice yield for a variety of produce. Method: Six juicers (Green Star Elite, Champion, NuWave vertical auger, LaLane centrifugal, Norwalk, Pure) were tested side by side for the production of carrot, apple, celery, spinach and a combination juice of carrot, celery, spinach, and lemon. Yields of 1 kg batches were measured four times for each combination of produce and juicer. The quality of juice was measured by testing a panel of enzyme activities in each juice. Enzyme activity of carrot and combination juices during storage up to 72 hours was also measured. Results: The Pure Juicer had the highest yields on all types of products tested, followed by the Norwalk and Green Star Elite, then the Champion, NuWave and LaLane juicers. The Pure Juicer and Norwalk both did significantly better at producing pure spinach juice than any other juicer. The Champion juicer had yields that were very similar to the Green Star Elite for carrots and combination juices. Enzyme activity was very comparable in all of the juicers. -

Prune Juice Concentrate
Additives in tobacco products Prune Juice Concentrate Additives are substances intentionally added to tobacco been classified by the International Agency for Research on products by tobacco industry in order to render toxic tobacco Cancer (a leading expert cancer organisation). Other toxic products palatable and acceptable to consumers. compounds that irritate the airways are also formed (e.g. acrolein or 2-furfural). Prunes are ripe plums that are dried. Concentrated prune juice is extracted from softened prunes. As a fruit extract, The sugars also produce acidic compounds, which make prune juice concentrate is very rich in sugars and is therefore it harder for the nicotine in the cigarette smoke to reach naturally sweet. the brain. This forces smokers to inhale deeper and to also consume more cigarettes to get their nicotine fix. Further- General uses more, the use of prune juice concentrate may be indirectly harmful due to the formation of compounds called aldehydes Prune juice concentrate has many uses in the food industry, (e.g. acetaldehyde), which can make cigarettes more addictive e.g. as a sweetener, colour and flavour enhancer, a binding by enhancing the addictive potential of nicotine. Aldehydes agent in cereal bars, and also as a ‘humectant’ to help keep are very reactive and produce compounds such as the subs- cakes and cookies moist. tance harman, which can also enhance addictiveness due to its mood-enhancing effect on the brain. Reported tobacco industry uses Prune juice concentrate is used to smoothen and mildly Prune juice concentrate (along with other extracts from either sweeten the smoke. It imparts a sweet taste making the the plum or prune) is reportedly used by tobacco manufac- smoke more palatable. -

New Research Suggests Drinking 100 Percent
NEW RESEARCH SUGGESTS consumption of 100 percent orange juice was not DRINKING 100 PERCENT associated with overweight or obesity in children. ORANGE JUICE IS ASSOCIATED WITH As part of the study, researchers analyzed data from the 2003-2006 National Health and Nutrition IMPROVED NUTRIENT Examination Survey (NHANES) and found that ADEQUACY AND DIET children who regularly consume 100 percent QUALITY AMONG CHILDREN orange juice tended to have significantly higher intakes of vitamin C, potassium, vitamin B6, folate, dietary fiber and magnesium than non- consumers. None of the children who consumed 100 percent orange juice were below the Estimated Average Requirement (EAR) for vitamin C, while nearly 30 percent of non- consumers were below the EAR. Furthermore, diet quality (as measured by the Healthy Eating Index (HEI-2005)) was significantly higher in those children consuming 100 percent orange juice than in non-consumers, as was intake of total fruit, fruit juice and whole fruit. "A growing body of research has painted a clear picture that enhanced nutrient intake and better diet quality are associated with drinking 100 percent orange juice in children," said study co- author Carol E. O'Neil, PhD, MPH, LDN, RD, School of Human Ecology, Louisiana State University Agricultural Center. "Our research adds One Hundred Percent Orange Juice May Play further support to the association between an Important Role in Supporting Intake of drinking 100 percent orange juice and higher Certain Under-consumed Nutrients intakes of five important nutrients--vitamin C, folate, magnesium, dietary fiber and potassium-- Orange juice may do more for children's diet and which are generally under-consumed by the U.S. -
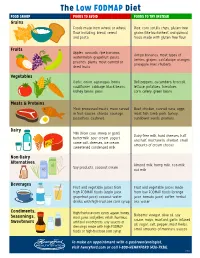
The Low FODMAP Diet
The Low FODMAP Diet FOOD GROUP FOODS TO AVOID FOODS TO TRY INSTEAD Grains Foods made from wheat or wheat Rice, corn tortilla chips, gluten-free flour including: bread, cereal grains (like buckwheat and quinoa), and pasta foods made with gluten-free flour Fruits Apples, avocado, ripe bananas, Unripe bananas, most types of watermelon, grapefruit, pears, berries, grapes, cantaloupe, oranges, peaches, plums, most canned or pineapple, kiwi, rhubarb dried fruits Vegetables Garlic, onion, asparagus, beets, Bell peppers, cucumbers, broccoli, cauliflower, cabbage, black beans, lettuce, potatoes, tomatoes, kidney beans, peas corn, celery, green beans Meats & Proteins Most processed meats, meat served Beef, chicken, canned tuna, eggs, in fruit sauces, chorizo, sausage, most fish, lamb, pork, turkey, pistachios, cashews sunflower seeds, peanuts Dairy Milk (from cow, sheep or goat), Dairy-free milk, hard cheeses, half buttermilk, sour cream, yogurt, and half, mozzarella, sherbet, small some soft cheeses, ice cream, amounts of cream cheese sweetened condensed milk Non-Dairy Alternatives Almond milk, hemp milk, rice milk, Soy products, coconut cream oat milk Beverages Fruit and vegetable juices from Fruit and vegetable juices made high FODMAP foods (apple juice, from low FODMAP foods (orange grapefruit juice), coconut water, juice, tomato juice), coffee, herbal drinks with high-fructose corn syrup tea, water Condiments, High-fructose corn syrup, agave, honey, Balsamic vinegar, olive oil, soy Seasonings, most jams and jellies, relish, hummus, sauce, mayo, mustard, garlic infused Sweeteners artificial sweeteners, any sauces or oil, sugar, salt, pepper, most herbs, dressings made with high FODMAP small amounts of marinara sauces foods or high-fructose corn syrup To make an appointment with a gastroenterologist, visit henryford.com or call 1-800-HENRYFORD (436-7936).