Photosensitisation Diseases of Animals Classification and A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tree of Life Marula Oil in Africa
HerbalGram 79 • August – October 2008 HerbalGram 79 • August Herbs and Thyroid Disease • Rosehips for Osteoarthritis • Pelargonium for Bronchitis • Herbs of the Painted Desert The Journal of the American Botanical Council Number 79 | August – October 2008 Herbs and Thyroid Disease • Rosehips for Osteoarthritis • Pelargonium for Bronchitis • Herbs of the Painted Desert • Herbs of the Painted Bronchitis for Osteoarthritis Disease • Rosehips for • Pelargonium Thyroid Herbs and www.herbalgram.org www.herbalgram.org US/CAN $6.95 Tree of Life Marula Oil in Africa www.herbalgram.org Herb Pharm’s Botanical Education Garden PRESERVING THE FULL-SPECTRUM OF NATURE'S CHEMISTRY The Art & Science of Herbal Extraction At Herb Pharm we continue to revere and follow the centuries-old, time- proven wisdom of traditional herbal medicine, but we integrate that wisdom with the herbal sciences and technology of the 21st Century. We produce our herbal extracts in our new, FDA-audited, GMP- compliant herb processing facility which is located just two miles from our certified-organic herb farm. This assures prompt delivery of freshly-harvested herbs directly from the fields, or recently HPLC chromatograph showing dried herbs directly from the farm’s drying loft. Here we also biochemical consistency of 6 receive other organic and wildcrafted herbs from various parts of batches of St. John’s Wort extracts the USA and world. In producing our herbal extracts we use precision scientific instru- ments to analyze each herb’s many chemical compounds. However, You’ll find Herb Pharm we do not focus entirely on the herb’s so-called “active compound(s)” at fine natural products and, instead, treat each herb and its chemical compounds as an integrated whole. -

Impact of Pesticide Use on Health in Developing Countries
Impact of pesticide use on health in developing countries Proceedings of a symposium held in Ottawa, Canada, 1 7-20 September 1990 IDRC CRDI International Development Research Centre Centre de recherches pour le devetoppement international 1 March 1993 Dear Reader/Librarian, IDRC is a public corporation created by the Canadian parliament in 1970 to help developing countries find viable solutions to their problems through research. At the 1992 Earth Summit, IDRC's mandate was broadened to emphasize sustainable development issues. As part of IDRC's strengthened commitment to global action and harüony, we are pleased to send you a complimentary copy of our most recent publication: The impact of pesticide use on health in developing countries (March 1993, 352 pages, 0-88936-560-1, $17.95). The first part of this book presents a brief survey of the global situation and the results of twelve epidemiological studies carried out by researchers from Africa, Latin America, Asia and the Middle East. These focus on poisonings resulting from organophosphates, herbicides, and pyrethroids. The second part illustrates the role of the process of development, production, spraying techniques and legislation in protecting the health of workers. A discussion of the benefits and modalities of access to pertinent information for the prevention of pesticide poisonings is provided in the third section. Finally, in the fourth section, consideration is given to the advantages and disadvantages of certain alternatives to the use of synthetic pesticides in agriculture and public health, such as botanical pesticides and integrated pest management strategies. We hope this book is a valuable addition to your collection. -

December 2012 Number 1
Calochortiana December 2012 Number 1 December 2012 Number 1 CONTENTS Proceedings of the Fifth South- western Rare and Endangered Plant Conference Calochortiana, a new publication of the Utah Native Plant Society . 3 The Fifth Southwestern Rare and En- dangered Plant Conference, Salt Lake City, Utah, March 2009 . 3 Abstracts of presentations and posters not submitted for the proceedings . 4 Southwestern cienegas: Rare habitats for endangered wetland plants. Robert Sivinski . 17 A new look at ranking plant rarity for conservation purposes, with an em- phasis on the flora of the American Southwest. John R. Spence . 25 The contribution of Cedar Breaks Na- tional Monument to the conservation of vascular plant diversity in Utah. Walter Fertig and Douglas N. Rey- nolds . 35 Studying the seed bank dynamics of rare plants. Susan Meyer . 46 East meets west: Rare desert Alliums in Arizona. John L. Anderson . 56 Calochortus nuttallii (Sego lily), Spatial patterns of endemic plant spe- state flower of Utah. By Kaye cies of the Colorado Plateau. Crystal Thorne. Krause . 63 Continued on page 2 Copyright 2012 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Utah Native Plant Society, PO Box 520041, Salt Lake Copyright 2012 Utah Native Plant Society. All Rights City, Utah, 84152-0041. www.unps.org Reserved. Calochortiana is a publication of the Utah Native Plant Society, a 501(c)(3) not-for-profit organi- Editor: Walter Fertig ([email protected]), zation dedicated to conserving and promoting steward- Editorial Committee: Walter Fertig, Mindy Wheeler, ship of our native plants. Leila Shultz, and Susan Meyer CONTENTS, continued Biogeography of rare plants of the Ash Meadows National Wildlife Refuge, Nevada. -

Aloe Ferox 117 Table 9: Phytochemical Constituents of Different Extracts of Aloe CIM- Sheetal Leaves 119
International Journal of Scientific & Engineering Research ISSN 2229-5518 1 Morphological, in vitro, Biochemical and Genetic Diversity Studies in Aloe species THESIS SUBMITTED TO OSMANIA UNIVERSITY FOR THE AWARD OF DOCTOR OF PHILOSOPHY IN GENETICS IJSER By B. CHANDRA SEKHAR SINGH DEPARTMENT OF GENETICS OSMANIA UNIVERSITY HYDERABAD - 500007, INDIA JULY, 2015 IJSER © 2018 http://www.ijser.org International Journal of Scientific & Engineering Research ISSN 2229-5518 2 DECLARATION The investigation incorporated in the thesis entitled “Morphological, in vitro, Biochemical and Genetic Diversity Studies in Aloe species’’ was carried out by me at the Department of Genetics, Osmania University, Hyderabad, India under the supervision of Prof. Anupalli Roja Rani, Osmania University, Hyderabad, India. I hereby declare that the work is original and no part of the thesis has been submitted for the award of any other degree or diploma prior to this date. IJSER Date: (Bhaludra Chandra Sekhar Singh) IJSER © 2018 http://www.ijser.org International Journal of Scientific & Engineering Research ISSN 2229-5518 3 DEDICATION I dedicateIJSER this work to my beloved and beautiful wife B. Ananda Sekhar IJSER © 2018 http://www.ijser.org International Journal of Scientific & Engineering Research ISSN 2229-5518 4 Acknowledgements This dissertation is an outcome of direct and indirect contribution of many people, which supplemented my own humble efforts. I like this opportunity to mention specifically some of them and extend my gratefulness to other well wisher, known and unknown. I feel extremely privileged to express my veneration for my superviosor Dr. Anupalli Roja Rani, Professor and Head, Department of Genetics, Osmania University, Hyderabad. Her whole- hearted co-operation, inspiration and encouragement rendered throughout made this in carrying out the research and writing of this thesis possible. -

Various Species, Mainly Aloe Ferox Miller and Its Hybrids)
European Medicines Agency Evaluation of Medicines for Human Use London, 5 July 2007 Doc. Ref: EMEA/HMPC/76313/2006 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) ASSESSMENT REPORT ON ALOE BARABADENSIS MILLER AND ALOE (VARIOUS SPECIES, MAINLY ALOE FEROX MILLER AND ITS HYBRIDS) Aloe barbadensis Miller (barbados aloes) Herbal substance Aloe [various species, mainly Aloe ferox Miller and its hybrids] (cape aloes) the concentrated and dried juice of the leaves, Herbal Preparation standardised; standardised herbal preparations thereof Pharmaceutical forms Herbal substance for oral preparation Rapporteur Dr C. Werner Assessor Dr. B. Merz Superseded 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 75 23 70 51 E-mail: [email protected] http://www.emea.europa.eu ©EMEA 2007 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged TABLE OF CONTENTS I. Introduction 3 II. Clinical Pharmacology 3 II.1 Pharmacokinetics 3 II.1.1 Phytochemical characterisation 3 II.1.2 Absorption, metabolism and excretion 4 II.1.3 Progress of action 5 II.2 Pharmacodynamics 5 II.2.1 Mode of action 5 • Laxative effect 5 • Other effects 7 II.2.2 Interactions 8 III. Clinical Efficacy 9 III.1 Dosage 9 III.2 Clinical studies 9 Conclusion 10 III.3 Clinical studies in special populations 10 III.3.1 Use in children 10 III.3.2. Use during pregnancy and lactation 10 III.3.3. Conclusion 13 III.4 Traditional use 13 IV. Safety 14 IV.1 Genotoxic and carcinogenic risk 14 IV.1.1 Preclinical Data 14 IV.1.2 Clinical Data 18 IV.1.3 Conclusion 20 IV.2 Toxicity 20 IV.3 Contraindications 21 IV.4 Special warnings and precautions for use 21 IV.5 Undesirable effects 22 IV.6 Interactions 22 IV.7 Overdose 23 V. -

Photodynamic Therapy for Cancer Role of Natural Products
Photodiagnosis and Photodynamic Therapy 26 (2019) 395–404 Contents lists available at ScienceDirect Photodiagnosis and Photodynamic Therapy journal homepage: www.elsevier.com/locate/pdpdt Review Photodynamic therapy for cancer: Role of natural products T Behzad Mansooria,b,d, Ali Mohammadia,d, Mohammad Amin Doustvandia, ⁎ Fatemeh Mohammadnejada, Farzin Kamaric, Morten F. Gjerstorffd, Behzad Baradarana, , ⁎⁎ Michael R. Hambline,f,g, a Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran b Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran c Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran d Department of Cancer and Inflammation Research, Institute for Molecular Medicine, University of Southern Denmark, 5000, Odense, Denmark e Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA 02114, USA f Department of Dermatology, Harvard Medical School, Boston, MA 02115, USA g Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA 02139, USA ARTICLE INFO ABSTRACT Keywords: Photodynamic therapy (PDT) is a promising modality for the treatment of cancer. PDT involves administering a Photodynamic therapy photosensitizing dye, i.e. photosensitizer, that selectively accumulates in tumors, and shining a light source on Photosensitizers the lesion with a wavelength matching the absorption spectrum of the photosensitizer, that exerts a cytotoxic Herbal medicine effect after excitation. The reactive oxygen species produced during PDT are responsible for the oxidation of Natural products biomolecules, which in turn cause cell death and the necrosis of malignant tissue. PDT is a multi-factorial process that generally involves apoptotic death of the tumor cells, degeneration of the tumor vasculature, stimulation of anti-tumor immune response, and induction of inflammatory reactions in the illuminated lesion. -

ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2008 ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Chemicals and Drugs Commons, and the Medical Sciences Commons Recommended Citation Fukuda, Yu , "ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters" (2008). Theses and Dissertations (ETD). Paper 345. http://dx.doi.org/10.21007/etd.cghs.2008.0100. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Interdisciplinary Program Research Advisor John D. Schuetz, Ph.D. Committee Linda Hendershot, Ph.D. James I. Morgan, Ph.D. Anjaparavanda P. Naren, Ph.D. Jie Zheng, Ph.D. DOI 10.21007/etd.cghs.2008.0100 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/345 ABCB6 IS A PORPHYRIN TRANSPORTER WITH A NOVEL TRAFFICKING SIGNAL THAT -

Natural Hydroxyanthraquinoid Pigments As Potent Food Grade Colorants: an Overview
Review Nat. Prod. Bioprospect. 2012, 2, 174–193 DOI 10.1007/s13659-012-0086-0 Natural hydroxyanthraquinoid pigments as potent food grade colorants: an overview a,b, a,b a,b b,c b,c Yanis CARO, * Linda ANAMALE, Mireille FOUILLAUD, Philippe LAURENT, Thomas PETIT, and a,b Laurent DUFOSSE aDépartement Agroalimentaire, ESIROI, Université de La Réunion, Sainte-Clotilde, Ile de la Réunion, France b LCSNSA, Faculté des Sciences et des Technologies, Université de La Réunion, Sainte-Clotilde, Ile de la Réunion, France c Département Génie Biologique, IUT, Université de La Réunion, Saint-Pierre, Ile de la Réunion, France Received 24 October 2012; Accepted 12 November 2012 © The Author(s) 2012. This article is published with open access at Springerlink.com Abstract: Natural pigments and colorants are widely used in the world in many industries such as textile dying, food processing or cosmetic manufacturing. Among the natural products of interest are various compounds belonging to carotenoids, anthocyanins, chlorophylls, melanins, betalains… The review emphasizes pigments with anthraquinoid skeleton and gives an overview on hydroxyanthraquinoids described in Nature, the first one ever published. Trends in consumption, production and regulation of natural food grade colorants are given, in the current global market. The second part focuses on the description of the chemical structures of the main anthraquinoid colouring compounds, their properties and their biosynthetic pathways. Main natural sources of such pigments are summarized, followed by discussion about toxicity and carcinogenicity observed in some cases. As a conclusion, current industrial applications of natural hydroxyanthraquinoids are described with two examples, carminic acid from an insect and Arpink red™ from a filamentous fungus. -

Illustrated Flora of East Texas Illustrated Flora of East Texas
ILLUSTRATED FLORA OF EAST TEXAS ILLUSTRATED FLORA OF EAST TEXAS IS PUBLISHED WITH THE SUPPORT OF: MAJOR BENEFACTORS: DAVID GIBSON AND WILL CRENSHAW DISCOVERY FUND U.S. FISH AND WILDLIFE FOUNDATION (NATIONAL PARK SERVICE, USDA FOREST SERVICE) TEXAS PARKS AND WILDLIFE DEPARTMENT SCOTT AND STUART GENTLING BENEFACTORS: NEW DOROTHEA L. LEONHARDT FOUNDATION (ANDREA C. HARKINS) TEMPLE-INLAND FOUNDATION SUMMERLEE FOUNDATION AMON G. CARTER FOUNDATION ROBERT J. O’KENNON PEG & BEN KEITH DORA & GORDON SYLVESTER DAVID & SUE NIVENS NATIVE PLANT SOCIETY OF TEXAS DAVID & MARGARET BAMBERGER GORDON MAY & KAREN WILLIAMSON JACOB & TERESE HERSHEY FOUNDATION INSTITUTIONAL SUPPORT: AUSTIN COLLEGE BOTANICAL RESEARCH INSTITUTE OF TEXAS SID RICHARDSON CAREER DEVELOPMENT FUND OF AUSTIN COLLEGE II OTHER CONTRIBUTORS: ALLDREDGE, LINDA & JACK HOLLEMAN, W.B. PETRUS, ELAINE J. BATTERBAE, SUSAN ROBERTS HOLT, JEAN & DUNCAN PRITCHETT, MARY H. BECK, NELL HUBER, MARY MAUD PRICE, DIANE BECKELMAN, SARA HUDSON, JIM & YONIE PRUESS, WARREN W. BENDER, LYNNE HULTMARK, GORDON & SARAH ROACH, ELIZABETH M. & ALLEN BIBB, NATHAN & BETTIE HUSTON, MELIA ROEBUCK, RICK & VICKI BOSWORTH, TONY JACOBS, BONNIE & LOUIS ROGNLIE, GLORIA & ERIC BOTTONE, LAURA BURKS JAMES, ROI & DEANNA ROUSH, LUCY BROWN, LARRY E. JEFFORDS, RUSSELL M. ROWE, BRIAN BRUSER, III, MR. & MRS. HENRY JOHN, SUE & PHIL ROZELL, JIMMY BURT, HELEN W. JONES, MARY LOU SANDLIN, MIKE CAMPBELL, KATHERINE & CHARLES KAHLE, GAIL SANDLIN, MR. & MRS. WILLIAM CARR, WILLIAM R. KARGES, JOANN SATTERWHITE, BEN CLARY, KAREN KEITH, ELIZABETH & ERIC SCHOENFELD, CARL COCHRAN, JOYCE LANEY, ELEANOR W. SCHULTZE, BETTY DAHLBERG, WALTER G. LAUGHLIN, DR. JAMES E. SCHULZE, PETER & HELEN DALLAS CHAPTER-NPSOT LECHE, BEVERLY SENNHAUSER, KELLY S. DAMEWOOD, LOGAN & ELEANOR LEWIS, PATRICIA SERLING, STEVEN DAMUTH, STEVEN LIGGIO, JOE SHANNON, LEILA HOUSEMAN DAVIS, ELLEN D. -

Phylogenetic Relationships of Ruteae (Rutaceae): New Evidence from the Chloroplast Genome and Comparisons with Non-Molecular Data
ARTICLE IN PRESS Molecular Phylogenetics and Evolution xxx (2008) xxx–xxx Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogenetic relationships of Ruteae (Rutaceae): New evidence from the chloroplast genome and comparisons with non-molecular data Gabriele Salvo a,*, Gianluigi Bacchetta b, Farrokh Ghahremaninejad c, Elena Conti a a Institute of Systematic Botany, University of Zürich, Zollikerstrasse 107, CH-8008 Zürich, Switzerland b Center for Conservation of Biodiversity (CCB), Department of Botany, University of Cagliari, Viale S. Ignazio da Laconi 13, 09123 Cagliari, Italy c Department of Biology, Tarbiat Moallem University, 49 Dr. Mofatteh Avenue, 15614 Tehran, Iran article info abstract Article history: Phylogenetic analyses of three cpDNA markers (matK, rpl16, and trnL–trnF) were performed to evaluate Received 12 December 2007 previous treatments of Ruteae based on morphology and phytochemistry that contradicted each other, Revised 14 July 2008 especially regarding the taxonomic status of Haplophyllum and Dictamnus. Trees derived from morpho- Accepted 9 September 2008 logical, phytochemical, and molecular datasets of Ruteae were then compared to look for possible pat- Available online xxxx terns of agreement among them. Furthermore, non-molecular characters were mapped on the molecular phylogeny to identify uniquely derived states and patterns of homoplasy in the morphological Keywords: and phytochemical datasets. The phylogenetic analyses determined that Haplophyllum and Ruta form Ruta reciprocally exclusive monophyletic groups and that Dictamnus is not closely related to the other genera Citrus family Morphology of Ruteae. The different types of datasets were partly incongruent with each other. The discordant phy- Phytochemistry logenetic patterns between the phytochemical and molecular trees might be best explained in terms of Congruence convergence in secondary chemical compounds. -
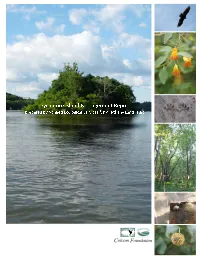
Final Si Management Report 10 06 10
Sycamore Island Management Report Prepared by Applied Ecological Services Inc. 1110 East Hector Street Conshohocken PA, 19428 For Allegheny Land Trust 409 Broad Street, Suite 206A Sewickley, PA 15143 This report is made possible by the generous support from TABLE OF CONTENTS 1. OVERVIEW 2. EXECUTIVE SUMMARY 3. PROJECT PHILOSOPHY AND APPROACH 4. SITE CONTEXT ‐ p.1 4.1 Location ‐ p.1 4.1. Geology and the Shaping of the Allegheny River and Surrounding Watershed ‐ p.1 4.2. Soils, Topography, and Drainage ‐ p.2 4.3. Ecology ‐ p.2 4.4. Cultural History ‐ p.3 4.5. Impacts of a Regulated River ‐ p.5 5. NATURAL RESOURCES INVENTORY, ECOLOGICAL ASSESSMENT AND MANAGEMENT RECOMMENDATIONS 5.1. Natural Community Mapping, Vegetation and Seedbank Studies ‐ p.7 5.2. Aquatic Species Surveys ‐ Fishes, Mollusks, and Macroinvertebrates ‐ p. 33 5.3. Vertebrate Species Surveys ‐ Reptiles, Amphibians, and Mammals ‐ p. 42 5.4. Avian Species Surveys ‐ p.48 5.5. Threatened and Endangered Species Survey and Existing Studies Review ‐ p. 57 5.6. Invasive Vegetative Species Management ‐ p. 63 5.7. Geotechnical Investigation ‐ p.68 5.8. Bathymetry Survey ‐ p.75 5.9. Human Use and Impact Study ‐ p. 76 6. TEST AND DEMONSTRATIONN PLOT TREATMENT AND MONITORING PLAN ‐ p.78 7. RECOMMENDATIONS FOR PUBLIC EDUCATION AND VOLUNTEER STEWARDSHIP ACTIVITIES ‐ p.85 8. TRAIL AND INTERPRETIVE SIGNAGE PLANS ‐ p.92 9. MANAGEMENT AND PRIORTIZATION STRATEGY FOR CARRYING OUT RECOMMENDATIONS ‐ p.96 10. REFERENCES ‐ p.106 APPENDICES A. Maps B. Soil Series C. Quadrat Datas D. T & E Species Search E. Invasive Vegetation Cut Sheets F. -

Cytotoxic Effect of Damnacanthal, Nordamnacanthal, Zerumbone and Betulinic Acid Isolated from Malaysian Plant Sources
International Food Research Journal 17: 711-719 (2010) Cytotoxic effect of damnacanthal, nordamnacanthal, zerumbone and betulinic acid isolated from Malaysian plant sources 1,*Alitheen, N.B., 2Mashitoh, A.R., 1Yeap, S.K., 3Shuhaimi, M., 4Abdul Manaf, A. and 2Nordin, L. 1Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia 2Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia 3Department. of Microbiology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia 4Faculty of Agriculture and Biotechnology, Universiti Darul Iman Malaysia, 20400 Kuala Terengganu, Terengganu, Malaysia Abstract: The present study was to evaluate the toxicity of damnacanthal, nordamnacanthal, betulinic acid and zerumbone isolated from local medicinal plants towards leukemia cell lines and immune cells by using MTT assay and flow cytometry cell cycle analysis. The results showed that damnacanthal significantly inhibited HL- 60 cells, CEM-SS and WEHI-3B with the IC50 value of 4.0 µg/mL, 8.0 µg/mL and 3.3 µg/mL, respectively. Nordamnacanthal and betulinic acid showed stronger inhibition towards CEM-SS and HL-60 cells with the IC50 value of 5.7 µg/mL and 5.0 µg/mL, respectively. In contrast, Zerumbone was demonstrated to be more toxic towards those leukemia cells with the IC50 value less than 10 µg/mL. Damnacanthal, nordamnacanthal and betulinic acid were not toxic towards 3T3 and PBMC compared to doxorubicin which showed toxicity effects towards 3T3 and PBMC with the IC50 value of 3.0 µg/mL and 28.0 µg/mL, respectively.