Dissecting a Neuron Network: FIB-SEM-Based 3D-Reconstruction of the Visual Neuropils in the Sea Spider Achelia Langi (Dohrn, 1881) (Pycnogonida) Lehmann Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurons and Glia
CHAPTER TWO Neurons and Glia INTRODUCTION THE NEURON DOCTRINE The Golgi Stain Cajal’s Contribution BOX 2.1 OF SPECIAL INTEREST: Advances in Microscopy THE PROTOTYPICAL NEURON The Soma The Nucleus Neuronal Genes, Genetic Variation, and Genetic Engineering BOX 2.2 BRAIN FOOD: Expressing One’s Mind in the Post-Genomic Era BOX 2.3 PATH OF DISCOVERY: Gene Targeting in Mice, by Mario Capecchi Rough Endoplasmic Reticulum Smooth Endoplasmic Reticulum and the Golgi Apparatus The Mitochondrion The Neuronal Membrane The Cytoskeleton Microtubules BOX 2.4 OF SPECIAL INTEREST: Alzheimer’s Disease and the Neuronal Cytoskeleton Microfilaments Neurofilaments The Axon The Axon Terminal The Synapse Axoplasmic Transport BOX 2.5 OF SPECIAL INTEREST: Hitching a Ride with Retrograde Transport Dendrites BOX 2.6 OF SPECIAL INTEREST: Intellectual Disability and Dendritic Spines CLASSIFYING NEURONS Classification Based on Neuronal Structure Number of Neurites Dendrites Connections Axon Length Classification Based on Gene Expression BOX 2.7 BRAIN FOOD: Understanding Neuronal Structure and Function with Incredible Cre GLIA Astrocytes Myelinating Glia Other Non-Neuronal Cells CONCLUDING REMARKS 23 © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION. 24 PART ONE FOUNDATIONS INTRODUCTION All tissues and organs in the body consist of cells. The specialized func- tions of cells and how they interact determine the functions of organs. The brain is an organ—to be sure, the most sophisticated and complex organ that nature has devised. But the basic strategy for unraveling its functions is no different from that used to investigate the pancreas or the lung. We must begin by learning how brain cells work individually and then see how they are assembled to work together. -
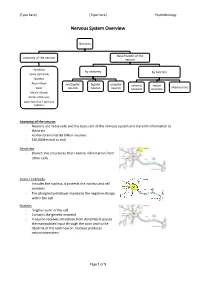
Nervous System Overview
[Type here] [Type here] Psychobiology Nervous System Overview Neurons classification of the anatomy of the neuron neuron dendrites by anatomy by function soma /cell body Nucleus Axon Hillock multipolar bipolar unipolar sensory motor interneurons Axon neuron neuron neuron neurons neurons Mylein Sheath Nodes of Ranvier Axon terminal / terminal buttons Anatomy of the neuron - Neurons are nerve cells and the basic unit of the nervous system and transmit information to the brain - Human brains has 86 billion neurons - 160,000km end to end Dendrites - Branch like structures that receive information from other cells Soma / Cell body - Includes the nucleus, it protects the nucleus and cell contents - The phospholipid bilayer maintains the negative charge within the cell Nucleus - ‘engine room’ of the cell - Contains the genetic material - If neuron receives simulation from dendrites it passes the manipulated input through the axon and to the dendrite of the next neuron. Nucleus produces neurotransmitters Page 1 of 5 [Type here] [Type here] Psychobiology Axon Hillock - The gatekeeper of transmission: this is where it is decided whether or not action potential is fired Axon terminals/ terminal buttons - Chemical messages are sent from these terminals - Gap between neurons are called synapses. Axon terminals are considered ‘pre-synaptic’ and dendrites are ‘post-synaptic’ Axon - Long nerve fibre - Transmits information to other neurons - Conducts the electrical signals from the cell body Myelin sheath - Coating that insulates the axon, composed of primarily of lipids (fats) - Allows for faster signalling - Produced by Schwan cells - Myelinated axons give some portions of the brain a white appearance Nodes of Ranvier - Bare axon - Allows the transmission to continue down the axon Classification of Neuron by Anatomy Multipolar Neuron Bipolar Neuron Unipolar Neuron - Long axon and lots of - 2 extensions from - 1 extension from the dendrites cell body cell body - (i.e. -

The Unipolar Neuron
The Unipolar Neuron The unipolar neuron is a sensory neuron and carries an electrical signal to the CNS. However, the anatomy of unipolar neurons is subject to different interpretations. As the unipolar neuron develops, there is a single protoplasmic process which extends from the neuron’s soma. This process splits immediately into two segments: 1) a proximal segment which enters the spinal cord; 2) a distal segment which extends out into the body and terminates in tissue as a “receptor”. Some refer to the two processes as “axons” while others refer to only the proximal process as an axon and the distal process as the dendrite. Saladin chooses the former while others (including myself!) choose the latter interpretation. Here is the critical issue. The receptor (i.e. dendrite) in the target tissue is stimulated and generates an action potential that moves towards the spinal cord (either in the dendrite or axon depending on which interpretation you choose). As the electrical signal approaches the soma, it does not need to create a local potential within the soma and the action potential continues to propagate the signal beyond the protoplasmic process of the soma. At the point of the soma’s protoplasmic process, the action potential does not enter the soma, but simply continues uninterrupted along the path to the spinal cord. Competing Definitions: 1. Unipolar neurons have but one process from the cell body. However, that single, very short, process splits into longer processes (a dendrite plus an axon). Unipolar neurons are sensory neurons - conducting impulses into the central nervous system. -

Normal Cells of the Cns
NORMAL CELLS OF THE CNS Color index: Slides.. Important ..Notes ..Extra.. Objectives: At the end of this lecture, you should describe the microscopic structure and the function of: 1- Neurons: Cell body (perikaryon). Processes: An axon and dendrites. 2- Neuroglia: Astrocytes. Oligodendrocytes. Microglia. Ependymal cells. Axon: only one Processes Neuron components Dendrites: one or more Cell body (Perikaryon) Types of neurons based on number of processes: Unipolar neuron Has one process only, that divides into two branches; (Pseudounipolar) one acts as a dendrite and the other as an axon. (rounded neuron) e.g. Mesencephalic nucleus of trigeminal nerve Not directly connected to the cell body and dorsal root (spinal) ganglion. Bipolar Neuron Has two processes (one arising from each pole of the cell body) (spindle-shaped neuron) One of them is the dendrite and the other is the axon. like having 2 necks e.g. retina & olfactory epithelium. Multipolar neuron: Stellate Neurons (star shape) Pyramidal Neurons (wide base) Pyriform Neurons Has one axon and multiple - The commonest type. - Distributed in motor area 4 - Pear-shaped dendrites. - Distributed in most areas of CNS of the cerebral cortex. e.g. Purkinje cells of cerebellar -Its outline is irregular in shape e.g. anterior horn cells of the -Neuroglial cells are much more cortex. number than neurons in the CNS spinal cord. they can divide and regenerate normally. Cell body (perikaryon) Cytoplasm: Cytoplasm with mitochondria and ribosomes and rough Nucleus: ER only in dendrites not in axons Single, usually central, rounded and Its main components include: vesicular with prominent nucleolus. Nissl Neuro- Micro- Golgi Mito- Centriole Pigments Other bodies filaments tubles apparatus chondria Depend on age Are * Are Most basophilic intermediate *lipofuscin patches of filaments adult pigment: in rough which are Are neurons old age bundled Endoplasmic found in have Some fat Reticulum together to the cell Surrounds *Melanin (rER) and form the Are only one and rudimentary pigments: in free neurofibrils. -

Guillain-Barre Syndrome (GBS)
Nervous tissue Anatomically Central nervous system (CNS) brain and spinal cord Peripheral nervous system (PNS) - cranial, spinal, and peripheral nerves - ganglia: nerve cell bodies outside the CNS Major cell types Neuron: nerve cell Supporting / Glial cells - Schwann cells, satellite cells (in PNS) - glia/neuroglia (in CNS) Neurone / Neuron Cell body Nucleus Cytoplasm (perikaryon) Process Axon Dendrites Axons (nerve fibers) Axon hillock Terminal boutons Dorsal root ganglia (DRG) nucleus ganglion/ganglia DRG neurons Basic neuron types Multipolar neuron Multiple dendrites Single axons Types: Interneurons Motor neurons Sympathetic neurons Bipolar neuron Single dendrite Single axon Types: Receptor neurons Vision Smell Balance Pseudo-unipolar neuron peripheral Single axon (Stem process) with stem 2 branches: Central process to spinal cord Peripheral process to terminal tissues (muscle, joints, skin et al) functionally: dendrite structurally: axon Type: Dorsal root ganglia (DRG neuron) central Pseudo-unipolar neuron Neuron: ultrastructure Rough endoplamic reticulum (rER) Nissl substance Cytoskeleton Microtubule Intermediate filaments: Neurofilaments Microfilaments: Actin Specialization of neuron/axon Cytoskeleton Axonal transport Neuron: ultrastructure rER: rough ER M: mitochondria L: lysosome G: Golgi Microscopic methods H & E (Hematoxylin and eosin) Nissl method Heavy metal impregnation Golgi, Cajal Thick sections / Spread preparations gold, silver: deposited in microtubules / neurofilaments Immunohistochemistry Microscopic methods: H & E -

Multilineage Differentiation Potential of CNS Cell Progenitors in a Recent
ssing oce & pr B o io i t e B f c Santacroce et al., J Bioproces Biotech 2014, 4:7 h o n l i a q n u DOI: 10.4172/2155-9821.1000186 r e u s o J Journal of Bioprocessing & Biotechniques ISSN: 2155-9821 Research Article Open Access Multilineage Differentiation Potential of CNS Cell Progenitors in a Recent Developed Gilthead Seabream (Sparus aurata L.) Nervous Model Maria Pia Santacroce1*, Antonella Tinelli2, Anna Selene Pastore1, Michele Colamonaco3 and Giuseppe Crescenzo3 1Unit of Aquaculture and Zooculture, Department of Veterinary Medicine, University of Bari “Aldo Moro”, Italy 2Unit of Pathology, Deptartment of Veterinary Medicine, University of Bari “Aldo Moro”, Italy 3Unit of Pharmacology and Toxicology, Department of Veterinary Medicine, University of Bari “Aldo Moro”, Italy Abstract Neural Progenitor Cells (NPCs) have gathered more and more attention in the field of Neural Stem Cells (NSCs). However, the multilineage differentiating behavior of these cells and their contribution to tissue regeneration, almost in lower vertebrate taxa, remain unknown. Since the early 1970s, many comparative studies have been performed using immunocytochemical screening on the brains of several vertebrate taxa, including teleosts, in order to identify these cells, even if the data are sometimes contrasting. This study aims: (1) to investigate in vitro the potential proliferative role of NPCs and Radial Glia Progenitors (RGP) in seabream neurogenesis; (2) to reveal the strict ability of fish NSCs to undertake the multilineage development and differentiation in neurons, astrocytes and oligodendrocytes. By the use of double Immunofluorescence (IF) analysis and phase contrast microscopy, we identified the multilineage differentiation and the exact cell morphology. -

Nerve Cell Impulses
• Localization of Certain Neurons Neurotransmitters Nerve Conduction by: Mary V. Andrianopoulos, Ph.D Clarification: Types of Neuron • There may be none, one, or many dendrites composing part of a neuron. • No dendrite = a unipolar neuron • One dendrite = bipolar neuron • More than one dendrite = multipolar neuron. Multipolar neuron Bipolar neuron Unipolar neuron Localization of Neuron types • Unipolar: – found in most of body's sensory neurons – dendrites are the exposed branches connected to receptors – axon carries the action potential in to the CNS – Examples: posterior root ganglia + cranial nerves – Usually: have peripheral + central connections Localization of Neuron types • Bipolar: – retina, sensory cochlear, vestibular ganglion • Multipolar: (fibers) brain + spinal cord – found as motor neurons and interneurons – neuronal tractsÆ CNS – peripheral nervesÆ PNS Size of Neurons + their localization • Golgi I: – Fiber tracts: brain + spinal cord (PNS + motor) – (i.e., Pyramidal tract + Purkinje cells) • Golgi II: – Cerebral + cerebellar cortex – Often inhibitory – Out number Golgi I – Star-shaped appearance 2° short dendrites Histology of the Nervous System A review of Cell types 1) Neurons - the functional cells of the nervous system 2) Neuroglia (glial cells) - Long described as supporting cells of the nervous system, there is also a functional interdependence of neuroglial cells and neurons a) astrocytes - anchor neurons to blood vessels, regulate the micro-environment of neurons, and regulate transport of nutrients and wastes to and from neurons b) microglia- are phagocytic to defend against pathogens and monitor the condition of neurons c) ependymal - line the fluid-filled cavities of the brain and spinal column and play a role in production, transport, and circulation of the CSF. -

The Nervous System
THE NERVOUS SYSTEM The nervous system primarily consists neurons and neuroglia. The functional unit of the nervous system are the neurons. A neuron can be defined as a nerve cell. The unique structure of neurons makes them specialized for receiving and transmitting electrical impulses throughout the body. The neuron acts like a miniature self-contained information processor. It receives inputs, processes information, and generates outputs. Neuron as the functional unit of nervous system was demonstrated by R.Y. Cajal while the nerve cell or the neuron was first described by Camillo Golgi in 1873. 3.1Structure of neuron The membrane, which surrounds the nerve cell, is made up of a double layer of lipid and contains protein molecules that play many important roles in transporting and blocking substances from coming in and out of the cell. A neuron has three main functional parts: the structure most associated with receiving signal is the dendrite, the body or soma , which accumulates signals coming from the input (dendrites) and which produces at the axonal hillock a series of bursts (impulses) when the accumulated signal reaches a critical threshold. These impulses are propagated to other neurons through an output called axon. The structure of neuron is described below: a) Neurocyton The cell body (soma or neurocyton or perikaryon) contains a granular cytoplasm due to the presence of basophilic granules called Nissl’s bodies. Nucleus is single and centrally located. Other cell organelles present are mitochondria, Golgi apparatus, ribosomes, endoplasmic reticulum , neurofibrillae and centrioles. The Nissl’s granules which are sometimes referred to as chromatophilic substances are composed of ribonucleoproteins are produced in the nucleus and play important role in. -
Axon (Nerve Fiber)—
Chapter 12 *Lecture PowerPoint Nervous Tissue *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The nervous system is one of great complexity • Nervous system is the foundation of our conscious experience, personality, and behavior • Neurobiology combines the behavioral and life sciences 11-2 Overview of the Nervous System • Expected Learning Outcomes – Describe the overall function of the nervous system. – Describe its major anatomical and functional subdivisions. 11-3 Overview of the Nervous System • Endocrine and nervous systems maintain internal coordination – Endocrine system: communicates by means of chemical messengers (hormones) secreted into to the blood – Nervous system: employs electrical and chemical means to send messages from cell to cell 12-4 Overview of the Nervous System • Nervous system carries out its task in three basic steps • Sense organs receive information about changes in the body and the external environment, and transmit coded messages to the spinal cord and the brain • Brain and spinal cord process this information, relate it to past experiences, and determine what response is appropriate to the circumstances • Brain and spinal cord issue commands to muscles and gland cells to carry out such a response 12-5 Overview of the Nervous System • Nervous system has two major anatomical subdivisions – Central nervous system (CNS) • Brain and spinal cord -

Neurons and Glia
Neurons and Glia INTRODUCTION THE NEURON DOCTRINE THEGOLGI STAIN CAJAL'SCONTRTBUTTON r Box 2.I O.fSpecial Interest: Advances in Microscopy THE PROTOTYPICAL NEURON THESOMA Ihe Nucleus r Box 2.2 Brain Food:Expressing One's Mind in the Post-GenomicEra RoughEndoplosmic Reticu/um SmoothEndoplosmic Reticulum ond the GolgiApporotus The Mitochondrion THE NEURONALMEMBRANE THE CYTOSKELETON Microtubules r Box 2.) Af SpecialInterest: Alzheimer's Disease and the Neuronal Cytoskeleton Miuofiloments Neurofloments THEAXON TheAxonTerminal Ihe Synopse AxoplosmicTronsport r Box 2.4 Of SpecialInterest: Hitching a Ride With RetrogradeTtansport DENDRITES r Box 2.5 Of SpecialInterest: Mental Retardationand Dendritic Spines r Box 2.6 Pathof Discovery:Spines and the StructuralBasis of Memory, by William Greenough CLASSIFYINGNEURONS CLASSIFICATIONBASED ON THE NUMBEROF NEURITES CLASSIFICATIONBASED ON DENDRITES CLASSIFICATIONBASED ON CONNECTIONS CLASSIFICATIONBASED ON AXON LENGTH CLASSIFICATIONBASED ON NEUROTRANSMITTER GLIA ASTROCYTES MYELINATINGGLIA OTHERNON-NEURONAL CELLS CONCLUDING REMARKS 24 CHAPTER 2 . NEURONSANDGLIA V INTRODUCTION All tissuesand organsin the body consistof cells.The specializedfunctions of cellsand how they interact determinethe functions of organs.The brain is an organ-to be sure, the most sophisticatedand complex organ that nature has devised.But the basicstrategy for unraveling its function is no different from that used to investigatethe pancreasor the lung. We must begin by learninghow brain cellswork individually and then seehow they are assembledto work together.In neuroscience,there is no need to sepa- rate mind from brain; once we fully understand the individual and con- certed actionsof brain cells,we will understandthe origins of our mental abilities.The organizationof this book reflectsthis "neurophilosophy."We start with the cells of the nervous system-their structure, function, and meansof communication.In later chapters,we will explorehow thesecells are assembledinto circuits that mediate sensation,perception, movement, speech,and emotion. -

Chapter 4 Central Nervous System and Sensory Organs
Chapter 4 Central Nervous System and Sensory Organs © David G. Ward © David Dendrites of Neuron Showing Dendritic Spines (Brighteld, Silver, x1880) 79 Sensory and Motor Neurons Cell Body Synaptic Bulb Dendrite / Receptor Axon (Central Process) Axon (Peripheral Process) Figure 4.1: Unipolar neuron (sensory). © David G. Ward. Dendrites Dendrites Cell Body Axon Hillock Synaptic Bulb Dendrites Axon Figure 4.2: Multipolar neuron (motor). © David G. Ward. 80 Chapter 4: Central Nervous System and Sensory Organs Communication Between Neurons Cell Body Dendrites Axon Cell Body Enlargement Synaptic Axon Synaptic Bulb Hillock Vesicles Synaptic Bulb Presynaptic Axon Membrane Postsynaptic Membrane Dendrite Synaptic Cleft Figure 4.3: Synaptic communication. © David G. Ward. Synaptic Bulb* Axon Dendrite Dendrite Synaptic Bulb* Synaptic Bulb Nucleus Axon Synaptic Vessicles *The synaptic bulbs are from other neurons communicating with this neuron. Presynaptic Membrane Figure 4.4: Mutlipolar neuron. Figure 4.5: Synaptic bulb. © David G. Ward. © David G. Ward. Chapter 4: Central Nervous System and Sensory Organs 81 Motor Neurons, Glial and Schwann Cells Multipolar Neurons Glial Cells Figure 4.6: Spinal multipolar neurons. © David G. Ward. Node Node Schwann Cell Axon Endoneurium Axon Myelin Sheath Figure 4.7: Myelinated neuron. © David G. Ward. Axon Node Axon Schwann Cell Schwann Cell 400x+ Axon Schwann Cell / Myelin Sheath Figure 4.8: Schwann cell. © David G. Ward. 82 Chapter 4: Central Nervous System and Sensory Organs Nerve and Schwann Cells Fascicle Perineurium Epineurium Perineurium Epineurium Fascicle Figure 4.9: Nerve cell, histology. © David G. Ward. Epineurium Perineurium Endoneurium Axons Perineurium Perineurium Fascicle Figure 4.10: Nerve. Figure 4.11: Nerve fascicle. -

Tutorial 3: Types of Neurons
Types of Neurons - Introduction 04/11/02 14:20 Tutorial 3: Types of Neurons Intro | Unipolar Neuron | Bipolar Neuron | Multipolar Neuron | Multipolar Interneuron Part 1: Image-Mapped Tutorial (Printable Version) Part 2: Matching Self-Test Part 3: Multiple-Choice Self-Test Return to main tutorial page Although all neurons contain the elements described in Tutorials 1 and 2, they can be classified according to the placement of the cell body relative to other portions of the neuron. The primary classification scheme is based on the number of branches that originate from the cell body. In this way, neurons are placed in one of three categories: unipolar (one branch), bipolar (two branches), or multipolar (multiple branches). Another way of distinguishing neurons is by overall shape, as affected significantly by the number and length of its branching dendrites and the length of its axon. The pattern of branching from the cell body affects the function of a neuron. These distinguishing characteristics of neurons are the topics of discussion in Tutorial 3. Suggestions for further study SUGGESTED READINGS: Baker, P.F. (1966, March). The nerve axon. Scientific American, 214(3), 74-82. Goldman, C.SS. & Bastiani, M.J. (1984, December). How embryonic nerve cells recognize one another. Scientific American, 251(6), 58-66. Halloway, M. (1992, January). Under construction. Temporary scaffolding guides nerves in the developing brain. Scientific American, 266(1), 25-26. Hubel, D.H. (1979, September). The brain. Scientific American, 241(3), 44-53. Kandel, E.R. (1979, September). Small systems of neurons. Scientific American, 241(3), 66-76. Nauta, W.J.