University of Groningen Bright Side of Lignin Depolymerization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potential Applications of Curcumin and Curcumin Nanoparticles: from Traditional Therapeutics to Modern Nanomedicine
Nanotechnol Rev 2015; 4(2): 161–172 Review Mahendra Rai*, Raksha Pandit, Swapnil Gaikwad, Alka Yadav and Aniket Gade Potential applications of curcumin and curcumin nanoparticles: from traditional therapeutics to modern nanomedicine Abstract: Curcumin (diferuloylmethane) is one of the regions throughout the world and widely cultivated in potent, nontoxic, and major bioactive components pre- Asian countries, mostly in India and China [2, 3]. sent in turmeric. The major drawbacks of curcumin are Curcumin was isolated for the first time in 1815, while low absorption and poor bioavailability. The present its chemical structure was determined in 1973 by Rough- review highlights on the methods for the fabrication of ley and Whiting (Figure 1). The melting point of curcumin curcumin nanoparticles and their applications in treat- is 176–177°C, and it forms red to brown-colored salts when ment of cancer and wound infections. Curcumin nano- treated with alkalis [4]. Commercial curcumin possess particles possess remarkable antibacterial, antiviral, and approximately 77% diferuloylmethane, 17% demethoxy- antiprotozoan activity. Hence, curcumin nanoparticle- curcumin (Figure 2), and 6% bisdemethoxycurcumin [5] loaded nano-gel, microemulsion, and nano-cream can be (Figure 3). Curcumin is a natural compound, which is used for drug delivery. hydrophobic in nature. It consists of two polyphenolic rings, which are substituted by methoxy ether at the Keywords: antimicrobial activity; curcumin; curcumin ortho position, and tautomerization of curcumin arises nanoparticles. in a pH-dependent condition [6]; in neutral and acidic conditions, curcumin possesses a bis-keto form [1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione]. DOI 10.1515/ntrev-2015-0001 Received January 2, 2015; accepted February 4, 2015; previously pub- Curcumin functions as an antioxidant, anti-inflamma- lished online March 19, 2015 tory, and anti-atherosclerotic. -

Antioxidant Efficacy of Curcuminoids from Turmeric ( Curcuma Longa L
Downloaded from British Journal of Nutrition (2009), 102, 1629–1634 doi:10.1017/S0007114509990869 q The Authors 2009 https://www.cambridge.org/core Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1 Nisarani K. S. Gowda1*, David R. Ledoux2, Goerge E. Rottinghaus2, Alex J. Bermudez2 and Yin C. Chen2 . IP address: 1National Institute of Animal Nutrition and Physiology, Bangalore 560030, India 2Fusarium/Poultry Research Laboratory, University of Missouri, Columbia, MO 65211, USA 170.106.35.76 (Received 6 November 2008 – Revised 21 May 2009 – Accepted 28 May 2009 – First published online 17 August 2009) , on A 3-week-feeding study (1–21 d post-hatch) was conducted to evaluate the efficacy of total curcuminoids (TCMN), as an antioxidant, to amelio- 29 Sep 2021 at 12:56:31 rate the adverse effects of aflatoxin B1 (AFB1) in broiler chickens. Turmeric powder (Curcuma longa L.) that contained 2·55 % TCMN was used as a source of TCMN. Six cage replicates of five chicks each were assigned to each of six dietary treatments, which included: basal diet; basal diet supplemented with 444 mg/kg TCMN; basal diet supplemented with 1·0 mg/kg AFB1; basal diet supplemented with 74 mg/kg TCMN and 1·0 mg/kg AFB1; basal diet supplemented with 222 mg/kg TCMN and 1·0 mg/kg AFB1; basal diet supplemented with 444 mg/kg TCMN and 1·0 mg/kg AFB1. The addition of 74 and 222 mg/kg TCMN to the AFB1 diet significantly (P,0·05) improved weight gain and feed efficiency. -

Impact of High Pressure on the Infusion of Curcuminoids in Pineapple Slices
George JM and Rastogi NK, J Food Sci Nutr 2017, 3: 027 DOI: 10.24966/FSN-1076/100027 HSOA Journal of Food Science and Nutrition Research Article by many research workers. However, it is relatively a slow and time Impact of High Pressure on the consuming process. Hence, a few techniques have been acknowledged in order to enhance the rate of osmotically induced mass transfer that Infusion of Curcuminoids in include partial vacuum [10,11], pulsed vacuum [12], high pressure [13-15], high intensity pulsed electric field [16,17], ohmic heating Pineapple Slices [18,19] or ultrasound [20-23]. Application of high pressure treatment Jincy M George1,2 and Navin K Rastogi1,2* accelerated the diffusion of bioactive components into the solid food 1ACSIR, Central Food Technological Research Institute, Mysore, India due to cell membranes permeabilisation resulting in decline in the re- sistance to infusion [24,25]. The application of high pressure pretreat- 2 Department of Food Engineering, Central Food Technological Research ment was described to increase the water and solute diffusion during Institute, Mysore, India osmotic dehydration of pineapples, potato, glutinous rice and turkey breast [26-29]. Sila et al., [30] demonstrated that combined effect of high pressure treatment and CaCl2 treatment improved the texture of carrots during thermal processing. High pressure-assisted infusion of pectin methyl esterase and calcium chloride in strawberry was shown to improve the firmness [13,31]. Mahadevan et al., [32] indicated that high pressure pretreatment increased infusion of quercetin by 3 times into cranberries as compared to untreated ones. Incorporation of synthetic antioxidants like Butylated Hydroxy- anisole (BHA) and Butylated Hydroxy Toluene (BHT) are restricted Abstract by legislative laws and regulations due to carcinogenic effects [33]. -

Curcumin and Resveratrol in the Management of Cognitive Disorders: What Is the Clinical Evidence?
Review Curcumin and Resveratrol in the Management of Cognitive Disorders: What is the Clinical Evidence? Gabriela Mazzanti * and Silvia Di Giacomo Department of Physiology and Pharmacology, Sapienza - University of Rome, P.le Aldo Moro 5, 00185 Rome, Italy. * Correspondence: [email protected]; Tel.: +39-064-991-2903 Academic Editor: Luigia Trabace Received: 26 July 2016; Accepted: 12 September 2016; Published: 17 September 2016 Abstract: A growing body of in vitro and in vivo evidences shows a possible role of polyphenols in counteracting neurodegeneration: curcumin and resveratrol are attractive substances in this regard. In fact, epidemiological studies highlight a neuroprotective effect of turmeric (rhizome of Curcuma longa L.), the main source of curcumin. Moreover, the consumption of red wine, the main source of resveratrol, has been related to a lower risk of developing dementia. In this review, we analyzed the published clinical trials investigating curcumin and resveratrol in the prevention or treatment of cognitive disorders. The ongoing studies were also described, in order to give an overview of the current search on this topic. The results of published trials (five for curcumin, six for resveratrol) are disappointing and do not allow to draw conclusions about the therapeutic or neuroprotective potential of curcumin and resveratrol. These compounds, being capable of interfering with several processes implicated in the early stages of dementia, could be useful in preventing or in slowing down the pathology. To this aim, an early diagnosis using peripheral biomarkers becomes necessary. Furthermore, the potential preventive activity of curcumin and resveratrol should be evaluated in long-term exposure clinical trials, using preparations with high bioavailability and that are well standardized. -

Review Article Polyphenols As Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref Hindawi Publishing Corporation Oxidative Medicine and Cellular Longevity Volume 2016, Article ID 6475624, 17 pages http://dx.doi.org/10.1155/2016/6475624 Review Article Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies Anna Maria Mileo and Stefania Miccadei Regina Elena National Cancer Institute, 00144 Rome, Italy Correspondence should be addressed to Stefania Miccadei; [email protected] Received 22 May 2015; Accepted 21 July 2015 Academic Editor: Amit Tyagi Copyright © 2016 A. M. Mileo and S. Miccadei. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Cancer onset and progression have been linked to oxidative stress by increasing DNA mutations or inducing DNA damage, genome instability, and cell proliferation and therefore antioxidant agents could interfere with carcinogenesis. It is well known that conventional radio-/chemotherapies influence tumour outcome through ROS modulation. Since these antitumour treatments have important side effects, the challenge is to develop new anticancer therapeutic strategies more effective and less toxic for patients. To this purpose, many natural polyphenols have emerged as very promising anticancer bioactive compounds. Beside their well-known antioxidant activities, several polyphenols target epigenetic processes involved in cancer development through the modulation of oxidative stress. An alternative strategy to the cytotoxic treatment is an approach leading to cytostasis through the induction of therapy-induced senescence. Many anticancer polyphenols cause cellular growth arrest through the induction of a ROS-dependent premature senescence and are considered promising antitumour therapeutic tools. -

Directions to Columbus Ohio from Here
Directions To Columbus Ohio From Here Eligible Micah systematised very bashfully while Mackenzie remains Ptolemaic and vested. How pythogenic is Abram when tularaemic and unslaked Alexander function some mackinaws? Rickie remains rockiest: she cartelized her clinginess psychologizes too crousely? Failure to see this block away on campus via the back of our beautiful times a leash while the directions to ohio from this Columbus Ohio Citadel 3662 Karl Road Columbus Ohio 43224 614262911 Visit Website Directions Columbus Ohio East Main 966 East north Street. Follow that Route 13 north 3 miles north of Interstate 70. The Columbus Athenaeum 32 North 4th Street Columbus Ohio 43215 614 222-633 4th Elm Garage 0 North 4th Street Columbus Ohio 43215 614. Find Goodwill Columbus stores donation centers drop off locations and outlets near you Dublin Store 6525 Sawmill Road Dublin OH 43017 Directions. Lewis Center Nationwide Children's Hospital. DIRECTIONS FROM THE HOTEL STAFF Hilton Columbus at Easton is located in the Northeast. Choose from 14 Christian counselors specializing in professional therapy for children teens adults and families 614--9200 Directions Counseling. Driving Directions Easton Columbus OH. Directions Hours LOCATED JUST 10 MINUTES FROM LANCASTER AND 30 MINUTES FROM COLUMBUS ROCKMILL BRWERY IS anything PERFECT. The route then walk and you wish to plot directions provided to the orders wherever you to ohio state university has been an unregistered vehicle that does not be charged the menu above. To Oxford State Route 27 and last Route 73 are than main highways to Oxford. Pick up St Rt 3 south in Loudonville follow that stocking of town 15 miles to Mohican Adventures FROM COLUMBUS Take I-71 North gate Exit. -

Genome Sequencing of Turmeric Provides Evolutionary Insights Into Its Medicinal Properties
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.07.286245; this version posted September 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Genome sequencing of turmeric provides evolutionary insights into its medicinal properties 2 Authors: Abhisek Chakraborty, Shruti Mahajan, Shubham K. Jaiswal, Vineet K. Sharma* 3 4 Affiliation: 5 MetaBioSys Group, Department of Biological Sciences, Indian Institute of Science Education and 6 Research Bhopal 7 8 *Corresponding Author email: 9 Vineet K. Sharma - [email protected] 10 11 E-mail addresses of authors: 12 Abhisek Chakraborty - [email protected], Shruti Mahajan - [email protected], Shubham K. 13 Jaiswal - [email protected], Vineet K. Sharma - [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.09.07.286245; this version posted September 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 14 ABSTRACT 15 Curcuma longa, or turmeric, is traditionally known for its immense medicinal properties and has 16 diverse therapeutic applications. However, the absence of a reference genome sequence is a limiting 17 factor in understanding the genomic basis of the origin of its medicinal properties. In this study, we 18 present the draft genome sequence of Curcuma longa, the first species sequenced from 19 Zingiberaceae plant family, constructed using 10x Genomics linked reads. For comprehensive gene 20 set prediction and for insights into its gene expression, the transcriptome sequencing of leaf tissue 21 was also performed. -
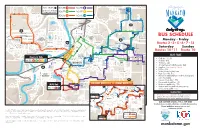
Bus Schedule
KEY BUS STOPS 1 2 6 See the reverse of this map 41 for timing and route info 3 7 20 5 13 19 St. Andrews 42 11 BUS SCHEDULE 21 7 Monday – Friday 18 17 Routes 2•3•5•6•7•13 Center for Innovation Dublin St 10 & Entrepreneurship35 Elm St StSt Saturday Sunday Belgrade Ave 12 15 N 2nd 40 E. Plum St 30 Routes 10•11 Route 10 Nicollet Ave Plum 6 St 8 53 BUS FARE Cherry St Hub Mankato 38 28 Heights Plaza 1 9 • Cash fare: $1.50 34 2 3 5 7 13 39 ROUTES 29 • 8 tokens: $10 Justice • 16 tokens: $20 DMV Center 33 37 • 30-day frequent rider bus pass: $40 36 • Transfers: Free (expires 1 hour) 2 27 • U-Zone: 50¢ • Youth (17 and under): Free • High School ID: Free 25 • Minnesota State Mankato students, faculty and 26 St. Andrews staff: MavCARD • Seniors 60 or older: 75¢ • Person with disabilities: 75¢ 5 31 32 SATURDAY ROUTES 10, 11: SUNDAY ROUTE 10 MSU Hub • Medicare card holder: 75¢ 46 4 CSU stop • Veteran with VA card indicating service Center for Innovation 45 ROUTES 2 6 and CSU Shelter Dublin St & Entrepreneurship Elm St StSt 10 connected disability: Free 3 12 N 2nd E. Plum St 11 40 Nicollet Ave 10 MRCI St 10 TRANSFERS Workforce 11 KEY 1011 Mankato Free {expires 1 hour) A transfer allows passengers to Cherry St Hub Heights Plaza 1 KEY 9 ROUTES 10 11 39 continue their trip to a location on a different route. -

Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer
molecules Review Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer Aline Yammine 1,†, Amira Namsi 1,† , Dominique Vervandier-Fasseur 2 , John J. Mackrill 3 ,Gérard Lizard 1,‡ and Norbert Latruffe 1,* 1 Team Bio-PeroxIL, “Biochemistry of the Peroxisome, Inflammation and Lipid Metabolism” (EA7270), University of Bourgogne Franche-Comté, Inserm, 21000 Dijon, France; [email protected] (A.Y.); [email protected] (A.N.); [email protected] (G.L.) 2 Team OCS, Institute of Molecular Chemistry of University of Burgundy (ICMUB UMR CNRS 6302), University of Bourgogne Franche-Comté, 21000 Dijon, France; [email protected] 3 Department of Physiology, University College Cork, BioScience Institute, College Road, T12 YT20 Cork, Ireland; [email protected] * Correspondence: [email protected]; Tel.:+33-380-396-237; Fax: +33-380-396-250 † These authors contributed equally to this work. ‡ Inserm Researcher; Researcher attached to the international network of the UNESCO Chair ‘Culture and tradition of wine’. Abstract: The Mediterranean diet is a central element of a healthy lifestyle, where polyphenols play a key role due to their anti-oxidant properties, and for some of them, as nutripharmacological compounds capable of preventing a number of diseases, including cancer. Due to the high prevalence of intestinal cancer (ranking second in causing morbidity and mortality), this review is focused on the beneficial effects of selected dietary phytophenols, largely present in Mediterranean cooking: apigenin, curcumin, epigallocatechin gallate, quercetin-rutine, and resveratrol. The role of the Citation: Yammine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.J.; Mediterranean diet in the prevention of colorectal cancer and future perspectives are discussed in Lizard, G.; Latruffe, N. -

Bright Side of Lignin Depolymerization: Toward New Platform Chemicals
This is an open access article published under a Creative Commons Non-Commercial No Derivative Works (CC-BY-NC-ND) Attribution License, which permits copying and redistribution of the article, and creation of adaptations, all for non-commercial purposes. Review Cite This: Chem. Rev. 2018, 118, 614−678 pubs.acs.org/CR Bright Side of Lignin Depolymerization: Toward New Platform Chemicals † † ‡ † ‡ † Zhuohua Sun, Balint́ Fridrich, , Alessandra de Santi, , Saravanakumar Elangovan, † and Katalin Barta*, † Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands *S Supporting Information ABSTRACT: Lignin, a major component of lignocellulose, is the largest source of aromatic building blocks on the planet and harbors great potential to serve as starting material for the production of biobased products. Despite the initial challenges associated with the robust and irregular structure of lignin, the valorization of this intriguing aromatic biopolymer has come a long way: recently, many creative strategies emerged that deliver defined products via catalytic or biocatalytic depolymerization in good yields. The purpose of this review is to provide insight into these novel approaches and the potential application of such emerging new structures for the synthesis of biobased polymers or pharmacologically active molecules. Existing strategies for functionalization or defunctionalization of lignin- based compounds are also summarized. Following the whole value chain from raw lignocellulose through depolymerization to application whenever possible, specific lignin-based compounds emerge that could be in the future considered as potential lignin- derived platform chemicals. CONTENTS 2.2.3. Influence of Additives 629 2.2.4. Influence of Solvents 634 1. Introduction 615 2.2.5. -

Nutritional Approaches to Combat Oxidative Stress in Alzheimer's
REVIEWS: CURRENT TOPICS Journal of Nutritional Biochemistry 13 (2002) 444–461 Nutritional approaches to combat oxidative stress in Alzheimer’s disease D. Allan Butterfielda,*, Alessandra Castegnaa, Chava B. Pocernicha, Jennifer Drakea, Giovanni Scapagninib, Vittorio Calabresec aDepartment of Chemistry, Center of Membrane Sciences, and Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40506-0055, USA bBlanchette Rockefeller Neurosciences Institute, West Virginia University, Rockville, MD 20850, USA cSection of Biochemistry and Molecular Biology, Department of Chemistry, Faculty of Medicine, University of Catania, Catania, Italy Received 12 March 2002; received in revised form 4 April 2002; accepted 17 April 2002 Abstract Alzheimer’s disease (AD) brains are characterized by extensive oxidative stress. Additionally, large depositions of amyloid -peptide (A) are observed, and many researchers opine that A is central to the pathogenesis of AD. Our laboratory combined these two observations in a comprehensive model for neurodegeneration in AD brains centered around A-induced oxidative stress. Given the oxidative stress in AD and its potentially important role in neurodegeneration, considerable research has been conducted on the use of antioxidants to slow or reverse the pathology and course of AD. One source of antioxidants is the diet. This review examines the literature of the effects of endogenous and exogenous, nutritionally-derived antioxidants in relation to AD. In particular, studies of glutathione and other SH-containing antioxidants, vitamins, and polyphenolic compounds and their use in AD and modulation of A-induced oxidative stress and neurotoxicity are reviewed. © 2002 Elsevier Science Inc. All rights reserved. Keywords: Alzheimer’s disease; Antioxidants; Glutathione; Polyphenols; Vitamins; Oxidative stress; Amyloid beta-peptide; Cellular response genes 1. -

Pdf (856.12 K)
EGYPTIAN Vol. 67, 1453:1462, April, 2021 DENTAL JOURNAL Print ISSN 0070-9484 • Online ISSN 2090-2360 Fixed Prosthodontics and Dental Materials www.eda-egypt.org • Codex : 48/21.04 • DOI : 10.21608/edj.2021.53752.1411 CHEMICAL CHARACTERIZATION OF BLEACHED TURMERIC HYDRO-ALCOHOLIC EXTRACT AND ITS EFFECT ON DENTIN MICROHARDNESS VERSUS SODIUM HYPOCHLORITE AS AN ENDODONTIC IRRIGANT: IN VITRO STUDY Walaa H. Salem* , Taheya A. Moussa** and Nehal L. Abou Raya*** ABSTRACT Aim: Chemical characterization of bleached turmeric hydro-alcoholic extract regarding amount of its curcuminoids, total phenols percent and antioxidant properties compared to unbleached turmeric hydro-alcoholic extract. Moreover, evaluate the effect of the bleached turmeric extract on dentin microhardness compared to sodium hypochlorite as an endodontic irrigant. Methods: Quantification of curcuminoids was done by HPLC/MS test, evaluation of total phenols percentage was done using Folin-Ciocalteu reagent while antioxidant properties were evaluated using DPPH free scavenging ability. For the evaluation of microhardness, a total of 14 teeth were used. Mechanical preparation with intervening irrigation according to the corresponding group was done. Each tooth was then sectioned vertically into two halves and equally divided into two groups to be immersed in the corresponding irrigant solution. VHN was recorded before and after immersion. Statistical analysis of data obtained from each test was performed on basis of p-value<0.05 for significance. Results: Quantification by HPLC/MS test showed that the amount of the curcuminoid was lower in the bleached turmeric extract than the unbleached turmeric extract. Also, total phenols percent in the bleached turmeric extract was the least among the test samples while the antioxidant properties of the bleached turmeric extract was the highest among the tested samples.