Eudocimus Albus (American White Ibis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

156 Glossy Ibis
Text and images extracted from Marchant, S. & Higgins, P.J. (co-ordinating editors) 1990. Handbook of Australian, New Zealand & Antarctic Birds. Volume 1, Ratites to ducks; Part B, Australian pelican to ducks. Melbourne, Oxford University Press. Pages 953, 1071-1 078; plate 78. Reproduced with the permission of Bird life Australia and Jeff Davies. 953 Order CICONIIFORMES Medium-sized to huge, long-legged wading birds with well developed hallux or hind toe, and large bill. Variations in shape of bill used for recognition of sub-families. Despite long legs, walk rather than run and escape by flying. Five families of which three (Ardeidae, Ciconiidae, Threskiornithidae) represented in our region; others - Balaenicipitidae (Shoe-billed Stork) and Scopidae (Hammerhead) - monotypic and exclusively Ethiopian. Re lated to Phoenicopteriformes, which sometimes considered as belonging to same order, and, more distantly, to Anseriformes. Behavioural similarities suggest affinities also to Pelecaniformes (van Tets 1965; Meyerriecks 1966), but close relationship not supported by studies of egg-white proteins (Sibley & Ahlquist 1972). Suggested also, mainly on osteological and other anatomical characters, that Ardeidae should be placed in separate order from Ciconiidae and that Cathartidae (New World vultures) should be placed in same order as latter (Ligon 1967). REFERENCES Ligon, J.D. 1967. Occas. Pap. Mus. Zool. Univ. Mich. 651. Sibley, C. G., & J.E. Ahlquist. 1972. Bull. Peabody Mus. nat. Meyerriecks, A.J. 1966. Auk 83: 683-4. Hist. 39. van Tets, G.F. 1965. AOU orn. Monogr. 2. 1071 Family PLATALEIDAE ibises, spoonbills Medium-sized to large wading and terrestial birds. About 30 species in about 15 genera, divided into two sub families: ibises (Threskiornithinae) and spoonbills (Plataleinae); five species in three genera breeding in our region. -

January 2020.Indd
BROWN PELICAN Photo by Rob Swindell at Melbourne, Florida JANUARY 2020 Editors: Jim Jablonski, Marty Ackermann, Tammy Martin, Cathy Priebe Webmistress: Arlene Lengyel January 2020 Program Tuesday, January 7, 2020, 7 p.m. Carlisle Reservation Visitor Center Gulls 101 Chuck Slusarczyk, Jr. "I'm happy to be presenting my program Gulls 101 to the good people of Black River Audubon. Gulls are notoriously difficult to identify, but I hope to at least get you looking at them a little closer. Even though I know a bit about them, I'm far from an expert in the field and there is always more to learn. The challenge is to know the particular field marks that are most important, and familiarization with the many plumage cycles helps a lot too. No one will come out of this presentation an expert, but I hope that I can at least give you an idea what to look for. At the very least, I hope you enjoy the photos. Looking forward to seeing everyone there!” Chuck Slusarczyk is an avid member of the Ohio birding community, and his efforts to assist and educate novice birders via social media are well known, yet he is the first to admit that one never stops learning. He has presented a number of programs to Black River Audubon, always drawing a large, appreciative gathering. 2019 Wellington Area Christmas Bird Count The Wellington-area CBC will take place Saturday, December 28, 2019. Meet at the McDonald’s on Rt. 58 at 8:00 a.m. The leader is Paul Sherwood. -

Incubation Constancy in the Red-Winged Blackbird
INCUBATION CONSTANCY IN THE RED-WINGED BLACKBIRD LARRY C. HOLCOMB Avian incubation behavior is affected by a multitude of exogenous and en- dogenous factors. Kendeigh (1952, 196313) and Skutch (1962) reviewed in- cubation in many different orders of birds and discussed factors affecting the amount of time spent in incubation. Among workers recently reporting on incubation behavior in wild passerines are Prescott (1964)) Mumford (1964)) Erpino (1968)) Maxwell and Putnam (1972)) and Morton et al. (1972). In the Red-winged Blackbird (Ag e1 aius phoeniceus) , Nero (1956a, 195613) has published observations on female behavior during the repro- ductive cycle, but nothing was reported on the incubation constancy (per- cent of daylight hours spent on the nest). I have reported (Holcomb, 1968, 1970) that female Redwings incubated normal-sized artificial eggs a mean of 19.4 days before abandoning them. This is 8.4 days beyond the normal incubation period. The present study was designed to determine the incubation constancy in the egg-laying period, to discover if it increased each day as the incubation behavior developed, and to find if there was less incubation each day as females neared the day when eggs were abandoned in prolonged incubation. METHODS I studied the incubation behavior of Red-winged Blackbirds near Omaha, Nebraska, in 1968 and 1969. Birds were breeding in a variety of habitats, including weed, alfalfa, and clover fields, hedgerows, ditch banks, and marshes. I visited the nesting areas nearly every day, beginning in March and ending in August. Male Redwings generally arrived in early March and females soon afterward. -

Behavior and Attendance Patterns of the Fork-Tailed Storm-Petrel
BEHAVIOR AND ATTENDANCE PATTERNS OF THE FORK-TAILED STORM-PETREL THEODORE R. SIMONS Wildlife Science Group, Collegeof Forest Resources, University of Washington, Seattle, Washington 98195 USA ABSTRACT.--Behavior and attendance patterns of breeding Fork-tailed Storm-Petrels (Ocea- nodromafurcata) were monitored over two nesting seasonson the Barren Islands, Alaska. The asynchrony of egg laying and hatching shown by these birds apparently reflects the influence of severalfactors, including snow conditionson the breedinggrounds, egg neglectduring incubation, and food availability. Communication between breeding birds was characterized by auditory and tactile signals.Two distinct vocalizationswere identified, one of which appearsto be a sex-specific call given by males during pair formation. Generally, both adults were present in the burrow on the night of egg laying, and the male took the first incubation shift. Incubation shiftsranged from 1 to 5 days, with 2- and 3-day shifts being the most common. Growth parameters of the chicks, reproductive success, and breeding chronology varied considerably between years; this pre- sumably relates to a difference in conditions affecting the availability of food. Adults apparently responded to changes in food availability during incubation by altering their attendance patterns. When conditionswere good, incubation shifts were shorter, egg neglectwas reduced, and chicks were brooded longer and were fed more frequently. Adults assistedthe chick in emerging from the shell. Chicks became active late in the nestling stage and began to venture from the burrow severaldays prior to fledging. Adults continuedto visit the chick during that time but may have reducedthe amountof fooddelivered. Chicks exhibiteda distinctprefledging weight loss.Received 18 September1979, accepted26 July 1980. -

IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2
IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2 Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group Djerba Island, Tunisia, 14th - 18th November 2018 Editors: Jocelyn Champagnon, Jelena Kralj, Luis Santiago Cano Alonso and K. S. Gopi Sundar Editors-in-Chief, Special Publications, IUCN-SSC Stork, Ibis and Spoonbill Specialist Group K.S. Gopi Sundar, Co-chair IUCN Stork, Ibis and Spoonbill Specialist Group Luis Santiago Cano Alonso, Co-chair IUCN Stork, Ibis and Spoonbill Specialist Group Invited Editors for this issue Jocelyn Champagnon, Tour du Valat, Research Institute for the Conservation of Mediterranean Wetlands, Arles, France Jelena Kralj, Institute of Ornithology, Zagreb, Croatia Expert Review Board Hichem Azafzaf, Association “les Amis des Oiseaux » (AAO/BirdLife Tunisia), Tunisia Petra de Goeij, Royal NIOZ, the Netherlands Csaba Pigniczki, Kiskunság National Park Directorate, Hungary Suggested citation of this publication: Champagnon J., Kralj J., Cano Alonso, L. S. & Sundar, K. S. G. (ed.) 2019. Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group. IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2. Arles, France. ISBN 978-2-491451-00-4. Recommended Citation of a chapter: Marion L. 2019. Recent trends of the breeding population of Spoonbill in France 2012- 2018. Pp 19- 23. In: Champagnon J., Kralj J., Cano Alonso, L. S. & Sundar, K. S. G. (ed.) Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group. IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2. Arles, France. INFORMATION AND WRITING DISCLAIMER The information and opinions expressed in this publication belong to the authors. -

Natural History and Breeding Behavior of the Tinamou, Nothoprocta Ornata
THE AUK A QUARTERLY JOURNAL OF ORNITHOLOGY VoL. 72 APRIL, 1955 No. 2 NATURAL HISTORY AND BREEDING BEHAVIOR OF THE TINAMOU, NOTHOPROCTA ORNATA ON the high mountainous plain of southern Peril west of Lake Titicaca live three speciesof the little known family Tinamidae. The three speciesrepresent three different genera and grade in size from the small, quail-sizedNothura darwini found in the farin land and grassy hills about Lake Titicaca between 12,500 and 13,300 feet to the large, pheasant-sized Tinamotis pentlandi in the bleak country between 14,000 and 16,000 feet. Nothoproctaornata, the third species in this area and the one to be discussedin the present report, is in- termediate in size and generally occurs at intermediate elevations. In Peril we have encountered Nothoproctabetween 13,000 and 14,300 feet. It often lives in the same grassy areas as Nothura; indeed, the two speciesmay be flushed simultaneouslyfrom the same spot. This is not true of Nothoproctaand the larger tinamou, Tinamotis, for although at places they occur within a few hundred yards of each other, Nothoproctais usually found in the bunch grassknown locally as ichu (mostly Stipa ichu) or in a mixture of ichu and tola shrubs, whereas Tinamotis usually occurs in the range of a different bunch grass, Festuca orthophylla. The three speciesof tinamous are dis- tinguished by the inhabitants, some of whom refer to Nothura as "codorniz" and to Nothoproctaas "perdiz." Tinamotis is always called "quivia," "quello," "keu," or some similar derivative of its distinctive call. The hilly, almost treeless countryside in which Nothoproctalives in southern Peril is used primarily for grazing sheep, alpacas,llamas, and cattle. -

The Migration Strategy, Diet & Foraging Ecology of a Small
The Migration Strategy, Diet & Foraging Ecology of a Small Seabird in a Changing Environment Renata Jorge Medeiros Mirra September 2010 Thesis submitted for the degree of Doctor of Philosophy, Cardiff School of Biosciences, Cardiff University UMI Number: U516649 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U516649 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Declarations & Statements DECLARATION This work has not previously been accepted in substance for any degree and is not concurrently submitted in candidature for any degree. Signed j K>X).Vr>^. (candidate) Date: 30/09/2010 STATEMENT 1 This thasjs is being submitted in partial fulfillment of the requirements for the degree o f ..................... (insertMCh, MD, MPhil, PhD etc, as appropriate) Signed . .Ate .^(candidate) Date: 30/09/2010 STATEMENT 2 This thesis is the result of my own independent work/investigation, except where otherwise stated. Other sources are acknowledgedjjy explicit references. Signe .. (candidate) Date: 30/09/2010 STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter-library loan, and for the title and summary to be made available to outside organisations. -

The Cycle of the Common Loon (Brochure)
ADIRONDACK LOONS AND LAKES FOR MORE INFORMATION: NEED YOUR HELP! lthough the Adirondack Park provides A suitable habitat for breeding loons, the summering population in the Park still faces many challenges. YOU CAN HELP! WCS’ Adirondack Loon Conservation Program Keep Shorelines Natural: Help maintain ~The Cycle of the this critical habitat for nesting wildlife and 7 Brandy Brook Ave, Suite 204 for the quality of our lake water. Saranac Lake, NY 12983 Common Loon~ (518) 891-8872, [email protected] Out on a Lake? Keep your distance (~100 feet or more) from loons and other wildlife, www.wcs.org/adirondackloons so that you do not disturb them. The Wildlife Conservation Society’s Adirondack Going Fishing? Loon Conservation Program is dedicated to ∗ Use Non-Lead Fishing Sinkers and improving the overall health of the environment, Jigs. Lead fishing tackle is poisonous to particularly the protection of air and water loons and other wildlife when quality, through collaborative research and accidentally ingested. education efforts focusing on the natural history ∗ Pack Out Your Line. Invisible in the of the Common Loon (Gavia immer) and water, lost or cut fishing line can conservation issues affecting loon populations entangle loons and other wildlife, often and their aquatic habitats. with fatal results. THE WILDLIFE CONSERVATION SOCIETY IS Be an Environmentally Wise Consumer: GRATEFUL TO ITS COLLABORATORS FOR THEIR Many forms of environmental pollution SUPPORT OF THE LOON PROGRAM: result from the incineration of fossil Natural History Museum of the Adirondacks - fuels, primarily from coal-fired power The W!ld Center plants and vehicles, negatively affecting www.wildcenter.org A guide to the seasonal Adirondack ecosystems and their wild NYS Dept. -

Uropygial Gland in Birds
UROPYGIAL GLAND IN BIRDS Prepared by Dr. Subhadeep Sarker Associate Professor, Department of Zoology, Serampore College Occurrence and Location: • The uropygial gland, often referred to as the oil or preen gland, is a bilobed gland and is found at the dorsal base of the tail of most psittacine birds. • The uropygial gland is a median dorsal gland, one per bird, in the synsacro-caudal region. A half moon-shaped row of feather follicles of the upper median and major tail coverts externally outline its position. • The uropygial area is located dorsally over the pygostyle on the midline at the base of the tail. • This gland is most developed in waterfowl but very reduced or even absent in many parrots (e.g. Amazon parrots), ostriches and many pigeons and doves. Anatomy: • The uropygial gland is an epidermal bilobed holocrine gland localized on the uropygium of most birds. • It is composed of two lobes separated by an interlobular septum and covered by an external capsule. • Uropygial secretory tissue is housed within the lobes of the gland (Lobus glandulae uropygialis), which are nearly always two in number. Exceptions occur in Hoopoe (Upupa epops) with three lobes, and owls with one. Duct and cavity systems are also contained within the lobes. • The architectural patterns formed by the secretory tubules, the cavities and ducts, differ markedly among species. The primary cavity can comprise over 90% of lobe volume in the Oilbird (Steatornis caripensis) and some woodpeckers and pigeons. • The gland is covered by a circlet or tuft of down feathers called the uropygial wick in many birds. -
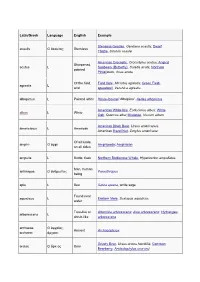
Dwarf Thistle, Cirsi
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Care of Fertile Eggs Prior to Incubation 1
CARE OF FERTILE EGGS PRIOR TO INCUBATION 1. Keep eggs at 50º - 60º F (room temperature). (DON’T PUT IN A REFRIGERATOR, IT IS TOO COLD!) 2. Store the eggs with the BIG end up in egg cartons. 3. You can store for 10 days after they have been laid before hatch rate decreases (50% hatch rate is a good rate). Incubation / hatching time begins once the eggs are placed in the incubator and brought up to 99 ½ºF. Count day 1 after first 24 hours. Incubation/hatching time for chickens are 21 days and 28 to 33 days for ducks. INCUBATOR SET UP 1. Place the incubator(s) away from any windows as the sunlight will magnify thru the plexi-glass cover making it too hot. 2. Set up the incubator four (4) hours prior to adding eggs; it will give the incubator an opportunity to Diagram #2 Round Corner regulate the water temperature in the reservoir. Diagram #1 Put water into both of the troughs on the square Square corner incubator incubator cornered incubators (see diagram #1). In the round corner model which has a larger and smaller trough, place water in the outside trough labeled “circulating” (see diagram #2). (Be sure that the incubator turns on at 99º and off at 100ºF.) 3. Place the plastic thermometer, simply lay it across the top of the eggs, (see diagram #5). It will basically ride on top of the eggs. 4. Cover the adjusting stem on top with paper cup taped to help eliminate the likelihood of the setting being accidentally changed. -

Class Three: Breeding
Class Three: Breeding WHERE DO SEABIRDS LAY THEIR EGGS? Nest or no nest? • Some species use no nesting material. E.g., the white tern lays a single egg on an open branch. • Some species use a very little bit of nesting material. E.g., the tufted puffin may use a few pieces of grass and a couple of feathers. • Some species build more substantial nests. E.g. kittiwakes cement their nests onto small cliff shelves by trampling mud and guano to form a base. And, some cormorants build large nests in trees from sticks and twigs. [email protected] www.seabirdyouth.org 1 White tern • Also called fairy tern. • Tropical seabird species. • Lays egg on branch or fork in tree. No nest. • Newly hatched chicks have well developed feet to hang onto the nesting-site. White Tern. © Pillot, via Creative Commons. On the coast or inland? • Most seabird species breed on the coast and offshore islands. • Some species breed fairly far inland, but still commute to the ocean to feed. E.g., kittlitz’s murrelets nest on scree slopes on coastal mountains, and parents may travel more than 70km to their feeding grounds. • Other species breed far inland and never travel to the ocean. E.g., double crested cormorants breed on the coast, but also on lakes in many states such as Minnesota. [email protected] www.seabirdyouth.org 2 NESTING HABITAT (1) Ground Some species breed on the ground. These species tend to breed in areas with little or no predation, such as offshore islands (e.g., terns and gulls) or in the Antarctic (e.g., penguins, albatross).