Physician Administered Drug Program
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®)
UnitedHealthcare® Commercial Medical Benefit Drug Policy Intravenous Iron Replacement Therapy (Feraheme®, Injectafer®, & Monoferric®) Policy Number: 2021D0088F Effective Date: July 1, 2021 Instructions for Use Table of Contents Page Community Plan Policy Coverage Rationale ....................................................................... 1 • Intravenous Iron Replacement Therapy (Feraheme®, Definitions ...................................................................................... 3 Injectafer®, & Monoferric®) Applicable Codes .......................................................................... 3 Background.................................................................................... 4 Benefit Considerations .................................................................. 4 Clinical Evidence ........................................................................... 5 U.S. Food and Drug Administration ............................................. 7 Centers for Medicare and Medicaid Services ............................. 8 References ..................................................................................... 8 Policy History/Revision Information ............................................. 9 Instructions for Use ....................................................................... 9 Coverage Rationale See Benefit Considerations This policy refers to the following intravenous iron replacements: Feraheme® (ferumoxytol) Injectafer® (ferric carboxymaltose) Monoferric® (ferric derisomaltose)* The following -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -
![Medical Synagis (CPT) [RSV-Igim], for Intramuscular Use, 50 (PA) Mg, Each)](https://docslib.b-cdn.net/cover/7463/medical-synagis-cpt-rsv-igim-for-intramuscular-use-50-pa-mg-each-1247463.webp)
Medical Synagis (CPT) [RSV-Igim], for Intramuscular Use, 50 (PA) Mg, Each)
CCHP Code Brand Name Description (PA) = Prior Authorization required Benefit Notes palivizumab (respiratory syncytial virus 90378 immune globulin Medical Synagis (CPT) [RSV-IgIM], for intramuscular use, 50 (PA) mg, each) lutetium lu 177, dotatate, therapeutic, Medical A9513 Lutathera 1 millicureie (PA) Medical A9590 Azedra Iodine I-131, iobenguane, 1 millicurie (PA) radium ra-223 dichloride, therapeutic, Medical A9606 Xofigo per microcurie (PA) in-line cartridge digestive enzyme for B4105 Relizorb Medical enteral feeding each COCAINE HCI NASAL SOL TOP ADMN 1 C9046 Goprelto Medical MG Medical C9047 Cablivi Injection, caplacizumab-yhdp, 1 mg (PA) Injection, romidepsin, non-lyophilized C9065 Medical (e.g. liquid), 1mg injection, belantamab mafodontin- C9069 Blenrep Medical blmf, 0.5 mg C9070 Monjuvi injection, tafasitamab-cxix, 2 mg Medical Medical C9071 Viltepso Injection, viltolarsen, 10 mg (PA) injection, immune globulin (asceniv), Medical C9072 Asceniv 500 mg (PA) Brexucabtagene autoleucel, up to 200 million autologous anti-cd19 car Medical C9073 Tecartus positive viable t cells, including (PA) leukapheresis and dose preparation procedures, per therapeutic dose Effective April 1, 2021 1 CCHP Code Brand Name Description (PA) = Prior Authorization required Benefit Notes prothrombin complex concentrate C9132 Kcentra (human), kcentra, per i.u. of factor ix Medical activity C9248 Cleviprex injection, clevidipine butyrate Medical C9250 Artiss artiss fibrin sealant Medical C9290 Exparel injection, bupivacaine liposome, 1 mg Medical C9293 Voraxaze -

Healthy U Medical Pharmacy Prior Authorization List
Below is the list of Medical Drug J-codes that require pre-service review for Healthy U. Please submit requests using the Medical Prior Auth Medical Electronic Request Form (select Medical Pharmacy from the drop down) or the PDF form that are available under Prior Authorization Forms, attach all necessary clinical documentation and submit to the Pharmacy Team by either fax to 801-213-1547 or by email: [email protected] If you have questions or need assistance regarding medical pharmacy please call for 833-981-0212 For questions regarding retail pharmacy please call RealRx pharmacy customer service: 855-856-5694 Procedure Code Description Portal Status 90378 RESPIRATORY SYNCYTIAL VIRUS IG IM 50 MG EA Not Covered 90626 TICK-BORNE ENCEPHALITIS VIRUS VACCINE, INACTIVATED; Not Covered 0.25 ML DOSAGE, FOR INTRAMUSCULAR USE 90627 TICK-BORNE ENCEPHALITIS VIRUS VACCINE, INACTIVATED; Not Covered 0.5 ML DOSAGE, FOR INTRAMUSCULAR USE 90671 PNEUMOCOCCAL CONJUGATE VACCINE, 15 VALENT Auth Required (PCV15), FOR INTRAMUSCUALR USE 90677 PNEUMOCOCCAL CONJUGATE VACCINE, 20 VALENT Auth Required (PCV20), FOR INTRAMUSCUALR USE 90758 ZAIRE EBOLAVIRUS VACCINE, LIVE, FOR INTRAMUSCUALR Not Covered USE 99601 HOME INFUSION/VISIT, 2 HRS Auth Required 99602 HOME INFUSION, EACH ADDTL HR Auth Required 0537T CAR-T THERAPY HRVG BLD DRV T LMPHCYT PR DAY Not Covered 0538T CAR-T THERAPY PREP BLOOD DERIVED T LMPHCYT FOR Not Covered TRANSPORTATION 0539T CAR-T THERAPY RECEIPT & PREP CAR-T CELLS FOR ADMIN Not Covered 0540T CAR-T THERAPY AUTOLOGOUS CELL ADMINISTRATION Not Covered A9276 DISPOSABLE SENSOR, CGM SYS Auth Required A9277 EXTERNAL TRANSMITTER, CGM Auth Required A9278 EXTERNAL RECEIVER, CGM SYS Auth Required A9513 LUTETIUM LU 177, DOTATATE, THERAPEUTIC, 1 MILLICURIE Auth Required 1 This Medical Pharmacy code list is updated regularly, and the Health Plan reserves the right to amend these policies and give notice in accordance with State and Federal requirements. -
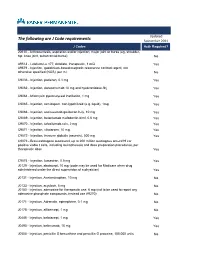
The Following Are J Code Requirements
Updated The following are J Code requirements September 2021 J Codes Auth Required? 20610 - Arthrocentesis, aspiration and/or injection; major joint or bursa (eg, shoulder, hip, knee joint, subacromial bursa) No A9513 - Lutetium Lu 177, dotatate, therapeutic, 1 mCi Yes A9579 - Injection, gadolinium-based magnetic resonance contrast agent, not otherwise specified (NOS), per ml No C9036 - Injection, patisiran, 0.1 mg Yes C9062 - Injection, daratumumab 10 mg and hyaluronidase-fihj Yes C9064 - Mitomycin pyelocalyceal instillation, 1 mg Yes C9065 - Injection, romidepsin, non-lypohilized (e.g. liquid), 1mg Yes C9066 - Injection, sacituzumab govitecan-hziy, 10 mg Yes C9069 - Injection, belantamab mafodontin-blmf, 0.5 mg Yes C9070 - Injection, tafasitamab-cxix, 2 mg Yes C9071 - Injection, viltolarsen, 10 mg Yes C9072 - Injection, immune globulin (asceniv), 500 mg Yes C9073 - Brexucabtagene autoleucel, up to 200 million autologous anti-cd19 car positive viable t cells, including leukapheresis and dose preparation procedures, per therapeutic dose Yes C9074 - Injection, lumasiran, 0.5 mg Yes J0129 - Injection, abatacept, 10 mg (code may be used for Medicare when drug administered under the direct supervision of a physician) Yes J0131 - Injection, Acetaminophen, 10 mg No J0133 - Injection, acyclovir, 5 mg No J0150 - Injection, adenosine for therapeutic use, 6 mg (not to be used to report any adenosine phosphate compounds, instead use A9270) No J0171 - Injection, Adrenalin, epinephrine, 0.1 mg No J0178 - Injection, aflibercept, 1 mg No J0485 - Injection, -

Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE Paclitaxel protein-bound particles, 1 mg THERAPEUTIC INJ CHEMOTHERAPY* J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 Tocilizumab, 1 mg SELF-INJECTABLE 0138-01) ACTEMRA INJECTION (50242-0136- J3262 Tocilizumab 200mg, 400mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 01, 50242-0137-01) J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB THERAPEUTIC INJ IMMUNIZATIONS dose schedule), for intramuscular use J0795 ACTHREL Corticorelin Ovine Triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J0791 ADAKVEO Crizanlizumab-tmca IV Solution THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY* -

Ferric Derisomaltose (Monoferric)
Clinical Policy: Ferric Derisomaltose (Monoferric) Reference Number: CP.PHAR.480 Effective Date: 06.01.20 Last Review Date: 05.21 Coding Implications Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Ferric derisomaltose (Monoferric™) injection is an iron replacement product. FDA Approved Indication(s) Monoferric is indicated for treatment of iron deficiency anemia (IDA) in adult patients: Who have intolerance to oral iron or have had unsatisfactory response to oral iron Who have non-hemodialysis dependent chronic kidney disease (NDD-CKD). Policy/Criteria Provider must submit documentation (including such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Monoferric is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Iron Deficiency Anemia with Chronic Kidney Disease (must meet all): 1. Diagnosis of IDA and CKD; 2. IDA is confirmed by either of the following: a. Transferrin saturation (TSAT) ≤ 30%; b. Serum ferritin ≤ 500 ng/mL; 3. If CKD does not require hemodialysis or peritoneal dialysis, oral iron therapy is not optimal due to any of the following: a. TSAT < 12%; b. Hgb < 7 g/dL; c. Symptomatic anemia; d. Severe or ongoing blood loss; e. Oral iron intolerance; f. Unable to achieve therapeutic targets with oral iron; g. Co-existing condition that may be refractory to oral iron therapy; 4. Dose does not exceed 1000 mg elemental iron (10 mL) per infusion/injection. -

Evolving Evidence-Based Treatment Guidelines for Iron Deficiency Anemia in Inflammatory Bowel Disease: Considerations in Managed Care
® SUPPLEMENT July 2021 THE AMERICAN JOURNAL OF MANAGED CARE® Vol. 27 • No. 11, Sup. Evolving Evidence-Based Treatment Guidelines for Iron Deficiency Anemia in Inflammatory Bowel Disease: Considerations in Managed Care HIGHLIGHTS › Diagnosis and Management of Iron Deficiency Anemia in Inflammatory Bowel Disease › Impact of Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease: Considerations in Managed Care › CE Sample Posttest Supplement to The American Journal of Managed Care® © 2021 Managed Care & Healthcare Communications, LLC Evolving Evidence-Based Treatment Guidelines for Iron Deficiency Anemia in Inflammatory Bowel Disease: Considerations in Managed Care Release date: July 15, 2021 Educational Objectives Expiration date: July 15, 2022 Upon completion of this activity, participants should be able to: • Examine the biologic pathways relevant to iron hemostasis in inflamma- Estimated time to complete activity: 2 hours tory bowel disease (IBD) as well as appropriate monitoring of iron status. Type of activity: Application • Compare iron replacement therapies, including risks and benefits, for the Medium: Print with internet-based posttest, evaluation, and request for treatment of patients with IBD and iron deficiency. credit • Explore the health-related quality of life and economic burden of iron Fee: Free deficiency anemia in relation to the evolving evidence-based treatment protocols for patients with IBD and iron deficiency to improve outcomes This activity is supported by an educational grant from American Regent. and efficiently allocate healthcare resources. Intended Audience Accreditation Statement Pharmacists and managed care professionals Pharmacy Times Continuing Education™ is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a Activity Overview provider of continuing pharmacy education. This activity is Iron deficiency anemia (IDA) is closely linked to inflammatory bowel approved for 2.0 contact hours (0.20 CEU) under the ACPE universal disease (IBD). -
![Extract from the Clinical Evaluation Report for [...]](https://docslib.b-cdn.net/cover/7731/extract-from-the-clinical-evaluation-report-for-3057731.webp)
Extract from the Clinical Evaluation Report for [...]
AusPAR Attachment 2 Extract from the Clinical Evaluation Report for Ferric derisomaltose Proprietary Product Name: Monofer Sponsor: Link Medical Products Pty Ltd T/A Link Pharmaceuticals First round report: 20 January 2017 Second round report: 23 June 2017 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Healthours, and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance), when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website <https://www.tga.gov.au>. About the Extract from the Clinical Evaluation Report • This document provides a more detailed evaluation of the clinical findings, extracted from the Clinical Evaluation Report (CER) prepared by the TGA. This extract does not include sections from the CER regarding product documentation or post market activities. • The words [Information redacted], where they appear in this document, indicate that confidential information has been deleted. • For the most recent Product Information (PI), please refer to the TGA website <https://www.tga.gov.au/product-information-pi>. -

Iron Deficiency Without Anaemia: a Diagnosis That Matters
Clinical Medicine 2021 Vol 21, No 2: 107–13 REVIEW Iron deficiency without anaemia: a diagnosis that matters Authors: Abdulrahman Al-Naseem,A Abdelrahman Sallam,A Shamim ChoudhuryA and Jecko ThachilB Iron deficiency anaemia (IDA) currently affects 1.2 billion females.3 Nevertheless, symptoms of anaemia such as fatigue people and iron deficiency without anaemia (IDWA) is at least can be present without anaemic haemoglobin levels.4 Recognising twice as common. IDWA is poorly recognised by clinicians IDWA as a clinical diagnosis is crucial to ensuring adequate despite its high prevalence, probably because of suboptimal management, especially for patients with chronic conditions such screening recommendations. Diagnosing IDWA relies on a as heart failure (HF) where IDWA can increase long-term mortality.5 ABSTRACT combination of tests, including haemoglobin and ferritin levels, as well as transferrin saturation. Although the causes Diagnostic definition of iron deficiency of iron deficiency may sometimes be obvious, many tend to be overlooked. Iron sufficiency throughout pregnancy Ferritin is an indicator of iron stores and is the most sensitive and is necessary for maternal and foetal health. Preoperative specific biomarker for assessing ID. The WHO defines low ferritin IDWA must be corrected to reduce the risk of transfusion and as levels <15 µg/L for adults and <12 µg/L for children.6 However, postoperative anaemia. Oral iron is the first-line treatment in clinical practice, when ferritin levels dip below 30 µg/L, ID can be for managing IDWA; however, intravenous supplementation ascertained.7 Ferritin is an acute-phase reactant that is increased should be used in chronic inflammatory conditions and when in serum during chronic inflammation. -

North Carolina Division of Health Benefits Physician Administered Drug Program Catalog
North Carolina Division of Health Benefits Physician Administered Drug Program Catalog North Carolina Division of Health Benefits Physician Administered Drug Program Catalog •Unless otherwise indicated, the catalog contains procedure codes representing drugs, biologics, devices and vaccines which are only covered for FDA approved indications. Covered indications that are not FDA approved are identified with **. •11 digit National Drug Codes (NDCs) are required to be billed along with their corresponding procedure code. Drugs and biologics must be classified as CMS covered outpatient drugs from a labeler/manufacturer participating in the Medicaid Drug Rebate Program (MDRP). •The Max Daily Units for radiopharmaceuticals represents one therapeutic dose or diagnostic dose. •The HCPCS Code effective date represents the date the HCPCS code was established •Procedure codes for covered devices and vaccines are not required to be from a rebating labeler/manufacturer as they are not classified as covered outpatient drugs. HCPCS HCPCS HCPCS Code Billing FDA Approved Indications Max Monthly Gender NDC Rebating Labeler Last Modified Category HCPCS Description Effective Brand Name Generic Name Max Daily Units Minimum Age Maximum Age Comments Code Unit (See Package Insert for full FDA approved indication descriptions) Units Restrictions Required Required Date Date Injection, amphotericin B lipid amphotericin B lipid complex Drugs J0287 10 mg 1/1/2003 Abelcet® Indicated for the treatment of invasive fungal infections in patients who are refractory to or intolerant of conventional amphotericin B therapy. 70 2,170 N/A N/A N/A Y Y 5/6/2019 complex, 10 mg injection aripiprazole extended-release Injection, aripiprazole, Indicated for the treatment of schizophrenia in adults. -

Challenge for COVID-19 Vaccines to Protect the New Zealand Population
Te ara tika o te hauora hapori Journal of the New Zealand Medical Association Vol 134 | No 1534 | 30 April 2021 Challenge for COVID-19 vaccines to protect the New Zealand population Making the Convention on the Rights of Persons with Disabilities real: our word is our bond Why is it so hard for general practice to provide COVID-19 vaccines? Parental use of physical What does abortion law Predictors of medicinal cannabis users’ punishment in a birth reform mean for primary care willingness to utilise a new prescription cohort practitioners in New Zealand? Medicinal Cannabis Scheme in New Zealand Te ara tika o te hauora hapori Publication Information published by the New Zealand Medical Association NZMJ Editor NZMA Chair Professor Frank Frizelle Dr Kate Baddock NZMJ Production Editor NZMA Communications Manager Richard Beer Madeleine Boles de Boers (Acting) Other enquiries to: To contribute to the NZMJ, first read: NZMA www.nzma.org.nz/journal/contribute PO Box 156 The Terrace © NZMA 2021 Wellington 6140 Phone: (04) 472 4741 Cover photo courtesy of the Ministry of Health To subscribe to the NZMJ, email [email protected] Subscription to the New Zealand Medical Journal is free and automatic to NZMA members. Private subscription is available to institutions, to people who are not medical practitioners, and to medical practitioners who live outside New Zealand. Subscription rates are below. All access to the NZMJ is by login and password, but IP access is available to some subscribers. Read our Conditions of access for subscribers for further information www.nzma.org.nz/journal/subscribe/conditions-of-access If you are a member or a subscriber and have not yet received your login and password, or wish to receive email alerts, please email: [email protected] The NZMA also publishes the NZMJ Digest.