Report and Evidence Tables
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urinary Incontinence: Impact on Long Term Care
Urinary Incontinence: Impact on Long Term Care Muhammad S. Choudhury, MD, FACS Professor and Chairman Department of Urology New York Medical College Director of Urology Westchester Medical Center 1 Urinary Incontinence: Overview • Definition • Scope • Anatomy and Physiology of Micturition • Types • Diagnosis • Management • Impact on Long Term Care 2 Urinary Incontinence: Definition • Involuntary leakage of urine which is personally and socially unacceptable to an individual. • It is a multifactorial syndrome caused by a combination of: • Genito urinary pathology. • Age related changes. • Comorbid conditions that impair normal micturition. • Loss of functional ability to toilet oneself. 3 Urinary Incontinence: Scope • Prevalence of Urinary incontinence increase with age. • Affects more women than men (2:1) up to age 80. • After age 80, both women and men are equally affected. • Urinary Incontinence affect 15% to 30% of the general population > 65 years. • > 50% of 1.5 million Long Term Care residents may be incontinent. • The cost to care for this group is >5 billion per year. • The total cost of care for Urinary Incontinence in the U.S. is estimated to be over $36 billion. Ehtman et al., 2012. 4 Urinary Incontinence: Impact on Quality of Life • Loss of self esteem. • Avoidance of social activity and interaction. • Decreased ability to maintain independent life style. • Increased dependence on care givers. • One of the most common reason for long term care placement. Grindley et al. Age Aging. 1998; 22: 82-89/Harris T. Aging in the eighties. NCHS # 121 1985. Noelker L. Gerontologist 1987; 27: 194-200. 5 Health related consequences of Urinary Incontinence • Increased propensity for fall/fracture. -

Cigna Home Delivery Pharmacy Drug List
Exclusive Cigna Home Delivery Pharmacy drug list If your prescription drug plan includes Exclusive Cigna Home Delivery Pharmacy, you are required to fill maintenance medication prescriptions (medications taken on an regular basis) through Cigna Home Delivery PharmacySM. Depending on your benefit plan, you are typically allowed three fills before you are required to use Cigna Home Delivery Pharmacy. At that time, if you choose not use Cigna Home Delivery Pharmacy, your plan will not cover the cost of your medication. To see real-time drug pricing, visit myCigna.com and use the Prescription Drug Price Quote tool to search for medications and compare pricing for your selected medications and lower-cost alternatives. The tool provides guidance on options you can discuss with your doctor and shows annual savings when you use Cigna Home Delivery Pharmacy. In addition to cost savings, Cigna Home Delivery Pharmacy offers valuable benefits, such as: • Licensed pharmacists available 24/7 • Up to a 90-day supply of medications in one fill • Standard delivery at no additional cost • Reminders if you forget to fill your prescriptions • Specialty medications, including those that require refrigeration and overnight delivery The following is a list of medications that apply to your Exclusive Cigna Home Delivery Pharmacy plan. Refer to your enrollment materials to understand the medications covered under your plan and to see if there are any requirements such as prior authorization (PA), quantity limits (QL) or Step Therapy (ST), as well as those requiring use of Cigna Home Delivery Pharmacy. If you have questions We’re here to help. Just call us at the toll-free number on your ID card and we will be happy to answer your questions. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

CMS Manual System Human Services (DHHS) Pub
Department of Health & CMS Manual System Human Services (DHHS) Pub. 100-07 State Operations Centers for Medicare & Provider Certification Medicaid Services (CMS) Transmittal 8 Date: JUNE 28, 2005 NOTE: Transmittal 7, of the State Operations Manual, Pub. 100-07 dated June 27, 2005, has been rescinded and replaced with Transmittal 8, dated June 28, 2005. The word “wound” was misspelled in the Interpretive Guidance section. All other material in this instruction remains the same. SUBJECT: Revision of Appendix PP – Section 483.25(d) – Urinary Incontinence, Tags F315 and F316 I. SUMMARY OF CHANGES: Current Guidance to Surveyors is entirely replaced by the attached revision. The two tags are being combined as one, which will become F315. Tag F316 will be deleted. The regulatory text for both tags will be combined, followed by this revised guidance. NEW/REVISED MATERIAL - EFFECTIVE DATE*: June 28, 2005 IMPLEMENTATION DATE: June 28, 2005 Disclaimer for manual changes only: The revision date and transmittal number apply to the red italicized material only. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual not updated.) (R = REVISED, N = NEW, D = DELETED) – (Only One Per Row.) R/N/D CHAPTER/SECTION/SUBSECTION/TITLE R Appendix PP/Tag F315/Guidance to Surveyors – Urinary Incontinence D Appendix PP/Tag F316/Urinary Incontinence III. FUNDING: Medicare contractors shall implement these instructions within their current operating budgets. IV. ATTACHMENTS: Business Requirements x Manual Instruction Confidential Requirements One-Time Notification Recurring Update Notification *Unless otherwise specified, the effective date is the date of service. -

The Effects of Oral Administration of the Novel Muscarinic Receptor
Choi et al. BMC Urology (2020) 20:41 https://doi.org/10.1186/s12894-020-00611-8 RESEARCH ARTICLE Open Access The effects of oral administration of the novel muscarinic receptor antagonist DA- 8010 on overactive bladder in rat with bladder outlet obstruction Jin Bong Choi1, Seung Hwan Jeon2, Eun Bi Kwon3, Woong Jin Bae4, Hyuk Jin Cho2, U-Syn Ha2, Sung-Hoo Hong2, Ji Youl Lee2 and Sae Woong Kim4* Abstract Background: DA-8010 is a novel compound developed for the treatment of overactive bladder (OAB) and urinary incontinence. The aims of this study were to investigate the effects of DA-8010 on OAB in a rat model. Methods: Study animals were divided into the following five groups of seven animals each: a sham-operated control group, a control group with partial bladder outlet obstruction (BOO) (OAB group), and three DA-8010 (doses of 0.3 mg/kg/day, 1 mg/kg/day, and 3 mg/kg/day, respectively) with partial BOO groups. Oral administration of the drugs was continued for 14 days after 2 weeks of partial BOO. After 4 weeks of partial BOO, cystometrography was performed in all groups. Additionally, pro-inflammatory cytokines, Rho-kinases, and histology of the bladder were analyzed. Results: There was a significant increase in the contraction interval and a decrease in contraction pressure in the 3 mg/kg/day DA-8010 group versus those in the OAB group. Rho kinase was also significantly decreased in the DA- 8010 3 mg/kg/day dosage treatment group. The increased ratio of collagen to smooth muscle after partial BOO was significantly attenuated in the DA-8010 3 mg/kg/day dosage group. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Anticholinergic Drugs Improve Symptoms but Increase Dry Mouth in Adults with Overactive Bladder Syndrome
Source of funding: Evid Based Nurs: first published as 10.1136/ebn.6.2.49 on 1 April 2003. Downloaded from Review: anticholinergic drugs improve symptoms but Health Research Council of Aotearoa increase dry mouth in adults with overactive bladder New Zealand. syndrome For correspondence: Jean Hay-Smith, Hay-Smith J, Herbison P,Ellis G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Dunedin School of Cochrane Database Syst Rev 2002;(3):CD003781 (latest version May 29 2002). Medicine, University of Otago, Dunedin, New QUESTION: What are the effects of anticholinergic drugs in adults with overactive Zealand. jean.hay-smith@ bladder syndrome? otago.ac.nz Data sources Parallel arm studies of anticholinergic drugs v placebo for overactive bladder syndrome in Studies were identified by searching the Cochrane adults at 12 days to 12 weeks* Incontinence Group trials register (to January 2002) Weighted event rates and reference lists of relevant papers. Anticholinergic Study selection Outcomes drugs Placebo RBI (95% CI) NNT (CI) Randomised or quasi-randomised controlled trials in Self reported cure or adults with symptomatic diagnosis of overactive bladder improvement (8 studies) 63% 45% 41% (29 to 54) 6 (5 to 8) syndrome, urodynamic diagnosis of detrusor overactiv- RRI (CI) NNH (CI) ity, or both, that compared an anticholinergic drug Dry mouth (20 studies) 36% 15% 138 (70 to 232) 5 (4 to 7) (given to decrease symptoms of overactive bladder) with Outcomes Weighted mean difference (CI) placebo or no treatment. Studies of darifenacin, emepronium bromide or carrageenate, dicyclomine Number of leakage episodes in 24 hours (9 − chloride, oxybutynin chloride, propiverine, propanthe- studies) 0.56 (–0.73 to –0.39) Number of micturitions in 24 hours (8 studies) −0.59 (–0.83 to –0.36) line bromide, tolterodine, and trospium chloride were Maximum cystometric volume (ml) (12 studies) 54.3 (43.0 to 65.7) included. -

Surgical Treatment of Urinary Incontinence in Men
Committee 13 Surgical Treatment of Urinary Incontinence in Men Chairman S. HERSCHORN (Canada) Members H. BRUSCHINI (Brazil), C.COMITER (USA), P.G RISE (France), T. HANUS (Czech Republic), R. KIRSCHNER-HERMANNS (Germany) 1121 CONTENTS I. INTRODUCTION VIII. TRAUMATIC INJURIES OF THE URETHRA AND PELVIC FLOOR II. EVALUATION PRIOR TO SURGICAL THERAPY IX. CONTINUING PEDIATRIC III. INCONTINENCE AFTER RADICAL PROBLEMS INTO ADULTHOOD: THE PROSTATECTOMY FOR PROSTATE EXSTROPHY-EPISPADIAS COMPLEX CANCER X. DETRUSOR OVERACTIVITY AND IV. INCONTINENCE AFTER REDUCED BLADDER CAPACITY PROSTATECTOMY FOR BENIGN DISEASE XI. URETHROCUTANEOUS AND V. SURGERY FOR INCONTINENCE IN RECTOURETHRAL FISTULAE ELDERLY MEN VI. INCONTINENCE AFTER XII. THE ARTIFICIAL URINARY EXTERNAL BEAM RADIOTHERAPY SPHINCTER (AUS) ALONE AND IN COMBINATION WITH SURGERY FOR PROSTATE CANCER XIII. SUMMARY AND RECOMMENDATIONS VII. INCONTINENCE AFTER OTHER TREATMENT FOR PROSTATE CANCER REFERENCES 1122 Surgical Treatment of Urinary Incontinence in Men S. HERSCHORN, H. BRUSCHINI, C. COMITER, P. GRISE, T. HANUS, R. KIRSCHNER-HERMANNS high-intensity focused ultrasound, other pelvic I. INTRODUCTION operations and trauma is a particularly challenging problem because of tissue damage outside the lower Surgery for male incontinence is an important aspect urinary tract. The artificial sphincter implant is the of treatment with the changing demographics of society most widely used surgical procedure but complications and the continuing large numbers of men undergoing may be more likely than in other areas and other surgery and other treatments for prostate cancer. surgical approaches may be necessary. Unresolved problems from pediatric age and patients with Basic evaluation of the patient is similar to other areas refractory incontinence from overactive bladders may of incontinence and includes primarily a clinical demand a variety of complex reconstructive surgical approach with history, frequency-volume chart or procedures. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -

Interstitial Cystitis/Painful Bladder Syndrome
What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse Contents What is interstitial cystitis/painful bladder syndrome (IC/PBS)? ............................................... 1 What are the signs of a bladder problem? ............ 2 What causes bladder problems? ............................ 3 Who gets IC/PBS? ................................................... 4 What tests will my doctor use for diagnosis of IC/PBS? ............................................................... 5 What treatments can help IC/PBS? ....................... 7 Points to Remember ............................................. 14 Hope through Research........................................ 15 Pronunciation Guide ............................................. 16 For More Information .......................................... 17 Acknowledgments ................................................. 18 What is interstitial cystitis/painful bladder syndrome (IC/PBS)? Interstitial cystitis*/painful bladder syndrome (IC/PBS) is one of several conditions that causes bladder pain and a need to urinate frequently and urgently. Some doctors have started using the term bladder pain syndrome (BPS) to describe this condition. Your bladder is a balloon-shaped organ where your body holds urine. When you have a bladder problem, you may notice certain signs or symptoms. *See page 16 for tips on how to say the words in bold type. 1 What are the signs of a bladder problem? Signs of bladder problems include ● Urgency. The feeling that you need to go right now! Urgency is normal if you haven’t been near a bathroom for a few hours or if you have been drinking a lot of fluids. -
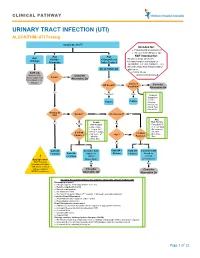
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate.