{Replace with the Title of Your Dissertation}
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish, Various Invertebrates
Zambezi Basin Wetlands Volume II : Chapters 7 - 11 - Contents i Back to links page CONTENTS VOLUME II Technical Reviews Page CHAPTER 7 : FRESHWATER FISHES .............................. 393 7.1 Introduction .................................................................... 393 7.2 The origin and zoogeography of Zambezian fishes ....... 393 7.3 Ichthyological regions of the Zambezi .......................... 404 7.4 Threats to biodiversity ................................................... 416 7.5 Wetlands of special interest .......................................... 432 7.6 Conservation and future directions ............................... 440 7.7 References ..................................................................... 443 TABLE 7.2: The fishes of the Zambezi River system .............. 449 APPENDIX 7.1 : Zambezi Delta Survey .................................. 461 CHAPTER 8 : FRESHWATER MOLLUSCS ................... 487 8.1 Introduction ................................................................. 487 8.2 Literature review ......................................................... 488 8.3 The Zambezi River basin ............................................ 489 8.4 The Molluscan fauna .................................................. 491 8.5 Biogeography ............................................................... 508 8.6 Biomphalaria, Bulinis and Schistosomiasis ................ 515 8.7 Conservation ................................................................ 516 8.8 Further investigations ................................................. -

Social Relationships in a Small Habitat-Dependent Coral Reef Fish: an Ecological, Behavioural and Genetic Analysis
ResearchOnline@JCU This file is part of the following reference: Rueger, Theresa (2016) Social relationships in a small habitat-dependent coral reef fish: an ecological, behavioural and genetic analysis. PhD thesis, James Cook University. Access to this file is available from: http://researchonline.jcu.edu.au/46690/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://researchonline.jcu.edu.au/46690/ Social relationships in a small habitat- dependent coral reef fish: an ecological, behavioural and genetic analysis Thesis submitted by Theresa Rueger, March 2016 for the degree of Doctor of Philosophy College of Marine and Environmental Science & ARC Centre of Excellence for Coral Reef Studies James Cook University Declaration of Ethics This research presented and reported in this thesis was conducted in compliance with the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th Edition, 2004 and the Qld Animal Care and Protection Act, 2001. The proposed research study received animal ethics approval from the JCU Animal Ethics Committee Approval Number #A1847. Signature ___31/3/2016___ Date i Acknowledgement This thesis was no one-woman show. There is a huge number of people who contributed, directly or indirectly, to its existence. I had amazing support during my field work, by fellow students and good friends Tiffany Sih, James White, Patrick Smallhorn-West, and Mariana Alvarez-Noriega. -

BREAK-OUT SESSIONS at a GLANCE THURSDAY, 24 JULY, Afternoon Sessions
2008 Joint Meeting (JMIH), Montreal, Canada BREAK-OUT SESSIONS AT A GLANCE THURSDAY, 24 JULY, Afternoon Sessions ROOM Salon Drummond West & Center Salons A&B Salons 6&7 SESSION/ Fish Ecology I Herp Behavior Fish Morphology & Histology I SYMPOSIUM MODERATOR J Knouft M Whiting M Dean 1:30 PM M Whiting M Dean Can She-male Flat Lizards (Platysaurus broadleyi) use Micro-mechanics and material properties of the Multiple Signals to Deceive Male Rivals? tessellated skeleton of cartilaginous fishes 1:45 PM J Webb M Paulissen K Conway - GDM The interopercular-preopercular articulation: a novel Is prey detection mediated by the widened lateral line Variation In Spatial Learning Within And Between Two feature suggesting a close relationship between canal system in the Lake Malawi cichlid, Aulonocara Species Of North American Skinks Psilorhynchus and labeonin cyprinids (Ostariophysi: hansbaenchi? Cypriniformes) 2:00 PM I Dolinsek M Venesky D Adriaens Homing And Straying Following Experimental Effects of Batrachochytrium dendrobatidis infections on Biting for Blood: A Novel Jaw Mechanism in Translocation Of PIT Tagged Fishes larval foraging performance Haematophagous Candirú Catfish (Vandellia sp.) 2:15 PM Z Benzaken K Summers J Bagley - GDM Taxonomy, population genetics, and body shape The tale of the two shoals: How individual experience A Key Ecological Trait Drives the Evolution of Monogamy variation of Alabama spotted bass Micropterus influences shoal behaviour in a Peruvian Poison Frog punctulatus henshalli 2:30 PM M Pyron K Parris L Chapman -

Kenyi Cichlid (Maylandia Lombardoi) Ecological Risk Screening Summary
Kenyi Cichlid (Maylandia lombardoi) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, April 2011 Revised, July 2018 Web Version, 8/3/2018 Photo: Ged~commonswiki. Public domain. Available: https://commons.wikimedia.org/wiki/File:Maylandia_lombardoi.jpg. (July 2018). 1 Native Range and Status in the United States Native Range From Kasembe (2017): “Endemic to Lake Malawi. Occurs at Mbenji Island and Nkhomo reef [Malawi].” From Froese and Pauly (2018): “Africa: Endemic to Mbenji Island, Lake Malawi [Malawi].” 1 Status in the United States This species has not been reported as introduced or established in the United States. This species is in trade in the United States. From Imperial Tropicals (2018): “Kenyi Cichlid (Pseudotropheus lombardoi) […] $ 7.99 […] UNSEXED 1” FISH” Means of Introductions in the United States This species has not been reported as introduced or established in the United States. Remarks There is taxonomic uncertainty concerning Maylandia lombardoi. Because it has recently been grouped in the genera Metriaclima and Pseudotropheus, these names were also used when searching for information in preparation of this assessment. From Kasembe (2017): “This species previously appeared on the IUCN Red List in the genus Maylandia but is now considered valid in the genus Metriaclima (Konings 2016, Stauffer et al. 2016).” From Seriously Fish (2018): “There is ongoing debate as to the true genus of this species, it having been variously grouped in both Maylandia and Metriaclima, as well as the currently valid Pseudotropheus. -
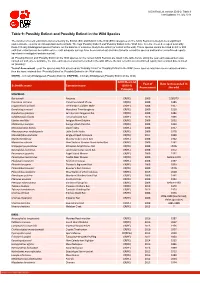
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2019-2: Table 9 Last Updated: 18 July 2019 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded in -

Indian and Madagascan Cichlids
FAMILY Cichlidae Bonaparte, 1835 - cichlids SUBFAMILY Etroplinae Kullander, 1998 - Indian and Madagascan cichlids [=Etroplinae H] GENUS Etroplus Cuvier, in Cuvier & Valenciennes, 1830 - cichlids [=Chaetolabrus, Microgaster] Species Etroplus canarensis Day, 1877 - Canara pearlspot Species Etroplus suratensis (Bloch, 1790) - green chromide [=caris, meleagris] GENUS Paretroplus Bleeker, 1868 - cichlids [=Lamena] Species Paretroplus dambabe Sparks, 2002 - dambabe cichlid Species Paretroplus damii Bleeker, 1868 - damba Species Paretroplus gymnopreopercularis Sparks, 2008 - Sparks' cichlid Species Paretroplus kieneri Arnoult, 1960 - kotsovato Species Paretroplus lamenabe Sparks, 2008 - big red cichlid Species Paretroplus loisellei Sparks & Schelly, 2011 - Loiselle's cichlid Species Paretroplus maculatus Kiener & Mauge, 1966 - damba mipentina Species Paretroplus maromandia Sparks & Reinthal, 1999 - maromandia cichlid Species Paretroplus menarambo Allgayer, 1996 - pinstripe damba Species Paretroplus nourissati (Allgayer, 1998) - lamena Species Paretroplus petiti Pellegrin, 1929 - kotso Species Paretroplus polyactis Bleeker, 1878 - Bleeker's paretroplus Species Paretroplus tsimoly Stiassny et al., 2001 - tsimoly cichlid GENUS Pseudetroplus Bleeker, in G, 1862 - cichlids Species Pseudetroplus maculatus (Bloch, 1795) - orange chromide [=coruchi] SUBFAMILY Ptychochrominae Sparks, 2004 - Malagasy cichlids [=Ptychochrominae S2002] GENUS Katria Stiassny & Sparks, 2006 - cichlids Species Katria katria (Reinthal & Stiassny, 1997) - Katria cichlid GENUS -

View/Download
CICHLIFORMES: Cichlidae (part 5) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 10.0 - 11 May 2021 Order CICHLIFORMES (part 5 of 8) Family CICHLIDAE Cichlids (part 5 of 7) Subfamily Pseudocrenilabrinae African Cichlids (Palaeoplex through Yssichromis) Palaeoplex Schedel, Kupriyanov, Katongo & Schliewen 2020 palaeoplex, a key concept in geoecodynamics representing the total genomic variation of a given species in a given landscape, the analysis of which theoretically allows for the reconstruction of that species’ history; since the distribution of P. palimpsest is tied to an ancient landscape (upper Congo River drainage, Zambia), the name refers to its potential to elucidate the complex landscape evolution of that region via its palaeoplex Palaeoplex palimpsest Schedel, Kupriyanov, Katongo & Schliewen 2020 named for how its palaeoplex (see genus) is like a palimpsest (a parchment manuscript page, common in medieval times that has been overwritten after layers of old handwritten letters had been scraped off, in which the old letters are often still visible), revealing how changes in its landscape and/or ecological conditions affected gene flow and left genetic signatures by overwriting the genome several times, whereas remnants of more ancient genomic signatures still persist in the background; this has led to contrasting hypotheses regarding this cichlid’s phylogenetic position Pallidochromis Turner 1994 pallidus, pale, referring to pale coloration of all specimens observed at the time; chromis, a name -

Cichlid Identification Slate
CICHLIDS of theMALERIIslands Mylochromis lateristriga Nimbochromis polystigma Protomelas sp. ‘oxyrhynchus mix’ Champsochromis caeruleus Dimidiochromis compressiceps Oreochromis squamipinnis Protomelas kirkii Protomelas similis Protomelas labridens Hemitilapia oxyrhynchus Tilapia rendalli Cyathochromis obliquidens Astatotilapia calliptera Rhamphochromis longiceps Serranochromis robustus Rhamphochromis esox Mylochromis sphaerodon Protomelas insignis Mylochromis mola Chilotilapia rhoadesii Dimidiochromis kiwinge Mylochromis anaphyrmus Stigmatochromis woodii Mylochromis melanonotus Pseudotropheus livingstonii Fossorochromis rostratus Metriaclima lanisticola Taeniolethrinops laticeps Trematocranus placodon Protomelas annectens Petrotilapia genalutea Oreochromis karongae Chilotilapia euchilus Oreochromis shiranus Copadichromis sp. ‘pictus maleri’ Corematodus taeniatus Tramitichromis brevis Tyrannochromis macrostoma Nimbochromis livingstonii Copadichromis insularis Protomelas fenestratus Protomelas ornatus Tropheops sp. ‘maleri yellow’ Mylochromis labidodon Caprichromis orthognathus Copadichromis atripinnis Melanochromis melanopterus Mylochromis incola Metriaclima sp. ‘patricki’ Metriaclima flavifemina Pseudotropheus sp. ‘burrower’ Mylochromis formosus Metriaclima barlowi Sciaenochromis sp. ‘nyassae’ Aulonocara sp. ‘stuartgranti maleri’ Tropheops sp. ‘maleri blue’ Pseudotropheus purpuratus Labidochromis vellicans Labeotropheus fuelleborni Metriaclima xanstomachus Pseudotropheus sp. ‘williamsi maleri’ Petrotilapia sp. ‘yellow chin’ Aristochromis -

Checklist of the Cichlid Fishes of Lake Malawi (Lake Nyasa)
Checklist of the Cichlid Fishes of Lake Malawi (Lake Nyasa/Niassa) by M.K. Oliver, Ph.D. ––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––– Checklist of the Cichlid Fishes of Lake Malawi (Lake Nyasa/Niassa) by Michael K. Oliver, Ph.D. Peabody Museum of Natural History, Yale University Updated 24 June 2020 First posted June 1999 The cichlids of Lake Malawi constitute the largest vertebrate species flock and largest lacustrine fish fauna on earth. This list includes all cichlid species, and the few subspecies, that have been formally described and named. Many–several hundred–additional endemic cichlid species are known but still undescribed, and this fact must be considered in assessing the biodiversity of the lake. Recent estimates of the total size of the lake’s cichlid fauna, counting both described and known but undescribed species, range from 700–843 species (Turner et al., 2001; Snoeks, 2001; Konings, 2007) or even 1000 species (Konings 2016). Additional undescribed species are still frequently being discovered, particularly in previously unexplored isolated locations and in deep water. The entire Lake Malawi cichlid metaflock is composed of two, possibly separate, endemic assemblages, the “Hap” group and the Mbuna group. Neither has been convincingly shown to be monophyletic. Membership in one or the other, or nonendemic status, is indicated in the checklist below for each genus, as is the type species of each endemic genus. The classification and synonymies are primarily based on the Catalog of Fishes with a few deviations. All synonymized genera and species should now be listed under their senior synonym. Nearly all species are endemic to L. Malawi, in some cases extending also into the upper Shiré River including Lake Malombe and even into the middle Shiré. -

Bending the Curve of Global Freshwater Biodiversity Loss
Forum Bending the Curve of Global Freshwater Biodiversity Loss: Downloaded from https://academic.oup.com/bioscience/advance-article-abstract/doi/10.1093/biosci/biaa002/5732594 by guest on 19 February 2020 An Emergency Recovery Plan DAVID TICKNER, JEFFREY J. OPPERMAN, ROBIN ABELL, MIKE ACREMAN, ANGELA H. ARTHINGTON, STUART E. BUNN, STEVEN J. COOKE, JAMES DALTON, WILL DARWALL, GAVIN EDWARDS, IAN HARRISON, KATHY HUGHES, TIM JONES, DAVID LECLÈRE, ABIGAIL J. LYNCH, PHILIP LEONARD, MICHAEL E. MCCLAIN, DEAN MURUVEN, JULIAN D. OLDEN, STEVE J. ORMEROD, JAMES ROBINSON, REBECCA E. THARME, MICHELE THIEME, KLEMENT TOCKNER, MARK WRIGHT, AND LUCY YOUNG Despite their limited spatial extent, freshwater ecosystems host remarkable biodiversity, including one-third of all vertebrate species. This biodiversity is declining dramatically: Globally, wetlands are vanishing three times faster than forests, and freshwater vertebrate populations have fallen more than twice as steeply as terrestrial or marine populations. Threats to freshwater biodiversity are well documented but coordinated action to reverse the decline is lacking. We present an Emergency Recovery Plan to bend the curve of freshwater biodiversity loss. Priority actions include accelerating implementation of environmental flows; improving water quality; protecting and restoring critical habitats; managing the exploitation of freshwater ecosystem resources, especially species and riverine aggregates; preventing and controlling nonnative species invasions; and safeguarding and restoring river connectivity. -

Species in Lake Malawi Dalitso R
The Chambo Restoration Strategic Plan Edited by Moses Banda Daniel Jamu Friday Njaya Maurice Makuwila Alfred Maluwa CHAPTER | Topic i The Chambo Restoration Strategic Plan Proceedings of the national workshop held on 13-16 May 2003 at Boadzulu Lakeshore Resort, Mangochi Edited by Moses Banda Daniel Jamu Friday Njaya Maurice Makuwila Alfred Maluwa 2005 Published by the WorldFish Center PO Box 500 GPO, 10670 Penang, Malaysia Banda, M., D. Jamu, F. Njaya, M. Makuwila and A. Maluwa (eds.) 2005. The Chambo Restoration Strategic Plan. WorldFish Center Conference Proceedings 71, 112 p. Perpustakaan Negara Malaysia. Cataloguing-in-Publication Data The chambo restoration plan / edited by Moses Banda ... [et al.]. ISBN 983-2346-36-3 1. Fisheries --Malawi--Conservation and restoration. 2. Fish-culture--Malawi--Management. I. Banda, Moses. 639.2096897 Cover photos by: C. Béné, R. Brummett and WorldFish photo collection ISBN 983-2346-36-3 WorldFish Center Contribution No. 1740 Printed by Printelligence, Penang, Malaysia. Reference to this publication should be duly acknowledged. The WorldFish Center is one of the 15 international research centers of the Consultative Group on International Agricultural Research (CGIAR) that has initiated the public awareness campaign, Future Harvest. ii WorldFish Center | Biodiversity, Management and Utilization of West African Fishes CHAPTER | Topic iii Contents Foreword v Acknowledgements vi Executive summary vii Introduction viii Official Opening Address by the Secretary for Natural Resources and Environment Affairs, Mr. G.C. Mkondiwa x Section 1: Review of the Chambo fisheries and biology ...................................................................................................... The status of the Chambo in Malawi: Fisheries and biology 1 M.C. Banda, G.Z. Kanyerere and B.B. -

Literature Review the Benefits of Wild Caught Ornamental Aquatic Organisms
LITERATURE REVIEW THE BENEFITS OF WILD CAUGHT ORNAMENTAL AQUATIC ORGANISMS 1 Submitted to the ORNAMENTAL AQUATIC TRADE ASSOCIATION October 2015 by Ian Watson and Dr David Roberts Durrell Institute of Conservation and Ecology [email protected] School of Anthropology and Conservation http://www.kent.ac.uk/sac/index.html University of Kent Canterbury Kent CT2 7NR United Kingdom Disclaimer: the views expressed in this report are those of the authors and do not necessarily represent the views of DICE, UoK or OATA. 2 Table of Contents Acronyms Used In This Report ................................................................................................................ 8 Executive Summary ............................................................................................................................... 10 Background to the Project .................................................................................................................... 13 Approach and Methodology ................................................................................................................. 13 Approach ........................................................................................................................................... 13 Literature Review Annex A ............................................................................................................ 13 Industry statistics Annex B .................................................................................................................... 15 Legislation