Integrative Physiology of Pneumonia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aalseth Aaron Aarup Aasen Aasheim Abair Abanatha Abandschon Abarca Abarr Abate Abba Abbas Abbate Abbe Abbett Abbey Abbott Abbs
BUSCAPRONTA www.buscapronta.com ARQUIVO 35 DE PESQUISAS GENEALÓGICAS 306 PÁGINAS – MÉDIA DE 98.500 SOBRENOMES/OCORRÊNCIA Para pesquisar, utilize a ferramenta EDITAR/LOCALIZAR do WORD. A cada vez que você clicar ENTER e aparecer o sobrenome pesquisado GRIFADO (FUNDO PRETO) corresponderá um endereço Internet correspondente que foi pesquisado por nossa equipe. Ao solicitar seus endereços de acesso Internet, informe o SOBRENOME PESQUISADO, o número do ARQUIVO BUSCAPRONTA DIV ou BUSCAPRONTA GEN correspondente e o número de vezes em que encontrou o SOBRENOME PESQUISADO. Número eventualmente existente à direita do sobrenome (e na mesma linha) indica número de pessoas com aquele sobrenome cujas informações genealógicas são apresentadas. O valor de cada endereço Internet solicitado está em nosso site www.buscapronta.com . Para dados especificamente de registros gerais pesquise nos arquivos BUSCAPRONTA DIV. ATENÇÃO: Quando pesquisar em nossos arquivos, ao digitar o sobrenome procurado, faça- o, sempre que julgar necessário, COM E SEM os acentos agudo, grave, circunflexo, crase, til e trema. Sobrenomes com (ç) cedilha, digite também somente com (c) ou com dois esses (ss). Sobrenomes com dois esses (ss), digite com somente um esse (s) e com (ç). (ZZ) digite, também (Z) e vice-versa. (LL) digite, também (L) e vice-versa. Van Wolfgang – pesquise Wolfgang (faça o mesmo com outros complementos: Van der, De la etc) Sobrenomes compostos ( Mendes Caldeira) pesquise separadamente: MENDES e depois CALDEIRA. Tendo dificuldade com caracter Ø HAMMERSHØY – pesquise HAMMERSH HØJBJERG – pesquise JBJERG BUSCAPRONTA não reproduz dados genealógicos das pessoas, sendo necessário acessar os documentos Internet correspondentes para obter tais dados e informações. DESEJAMOS PLENO SUCESSO EM SUA PESQUISA. -
IFM Innate Immunity Infographic
UNDERSTANDING INNATE IMMUNITY INTRODUCTION The immune system is comprised of two arms that work together to protect the body – the innate and adaptive immune systems. INNATE ADAPTIVE γδ T Cell Dendritic B Cell Cell Macrophage Antibodies Natural Killer Lymphocites Neutrophil T Cell CD4+ CD8+ T Cell T Cell TIME 6 hours 12 hours 1 week INNATE IMMUNITY ADAPTIVE IMMUNITY Innate immunity is the body’s first The adaptive, or acquired, immune line of immunological response system is activated when the innate and reacts quickly to anything that immune system is not able to fully should not be present. address a threat, but responses are slow, taking up to a week to fully respond. Pathogen evades the innate Dendritic immune system T Cell Cell Through antigen Pathogen presentation, the dendritic cell informs T cells of the pathogen, which informs Macrophage B cells B Cell B cells create antibodies against the pathogen Macrophages engulf and destroy Antibodies label invading pathogens pathogens for destruction Scientists estimate innate immunity comprises approximately: The adaptive immune system develops of the immune memory of pathogen exposures, so that 80% system B and T cells can respond quickly to eliminate repeat invaders. IMMUNE SYSTEM AND DISEASE If the immune system consistently under-responds or over-responds, serious diseases can result. CANCER INFLAMMATION Innate system is TOO ACTIVE Innate system NOT ACTIVE ENOUGH Cancers grow and spread when tumor Certain diseases trigger the innate cells evade detection by the immune immune system to unnecessarily system. The innate immune system is respond and cause excessive inflammation. responsible for detecting cancer cells and This type of chronic inflammation is signaling to the adaptive immune system associated with autoimmune and for the destruction of the cancer cells. -

Captive Orcas
Captive Orcas ‘Dying to Entertain You’ The Full Story A report for Whale and Dolphin Conservation Society (WDCS) Chippenham, UK Produced by Vanessa Williams Contents Introduction Section 1 The showbiz orca Section 2 Life in the wild FINgerprinting techniques. Community living. Social behaviour. Intelligence. Communication. Orca studies in other parts of the world. Fact file. Latest news on northern/southern residents. Section 3 The world orca trade Capture sites and methods. Legislation. Holding areas [USA/Canada /Iceland/Japan]. Effects of capture upon remaining animals. Potential future capture sites. Transport from the wild. Transport from tank to tank. “Orca laundering”. Breeding loan. Special deals. Section 4 Life in the tank Standards and regulations for captive display [USA/Canada/UK/Japan]. Conditions in captivity: Pool size. Pool design and water quality. Feeding. Acoustics and ambient noise. Social composition and companionship. Solitary confinement. Health of captive orcas: Survival rates and longevity. Causes of death. Stress. Aggressive behaviour towards other orcas. Aggression towards trainers. Section 5 Marine park myths Education. Conservation. Captive breeding. Research. Section 6 The display industry makes a killing Marketing the image. Lobbying. Dubious bedfellows. Drive fisheries. Over-capturing. Section 7 The times they are a-changing The future of marine parks. Changing climate of public opinion. Ethics. Alternatives to display. Whale watching. Cetacean-free facilities. Future of current captives. Release programmes. Section 8 Conclusions and recommendations Appendix: Location of current captives, and details of wild-caught orcas References The information contained in this report is believed to be correct at the time of last publication: 30th April 2001. Some information is inevitably date-sensitive: please notify the author with any comments or updated information. -

Greg J Sheehan, FWS Principal Deputy Director
Greg J Sheehan, FWS Principal Deputy Director Tue Jun 6, 2017 2:45pm FWS weekly check in meeting Video call: (b) (5), (b) (6) Where: AS/FWP Conference Room -- 3144 Calendar: Maureen Foster Created by: Tasha Robbins Who: Thomas Irwin, Charisa Morris, Maureen Foster, Richard Goeken, GregSheehan, Wendy Fink, Stephen Guertin, Catherine Gulac, Jason Larrabee,Tasha Robbins, Aurelia Skipwith, Marshall Critchfield, Heather Swift,Jim Kurth, Todd Willens, Roslyn Sellars, Zachariah Gambill, PegRomanik, Barbara Wainman 3pm *FWS weekly check in meeting Video call: (b) (5), (b) (6) Where: AS/FWP Conference Room -- 3144 Calendar: Greg Sheehan Created by: Roslyn Sellars Wed Jun 7, 2017 1pm OIG monthly status update meeting/conference call (Jim, Steve, Kathy Garrity, Keith Toomey, Charisa) Room 3357 Video call: (b) (5), (b) (6) Where: Dial: (b) (5) , Code: (b) (5) Calendar: Jim Kurth Created by: Roslyn Sellars Who: Casey Hammond, Stephen Guertin, Katherine Garrity, Jim Kurth, GregSheehan, Charisa Morris, Keith Toomey 2:30pm [Asst Directors/Regional Directors Only-No Deputies or Actings] - Weekly Directorate VTC: Transition Check-In--Room 3038 Video call: (b) (5), (b) (6) Calendar: Jim Kurth Created by: Thomas Irwin Who: [email protected], Tom Melius, [email protected], WandaCantrell, Edward Grace, [email protected], Henry Schlitzer,Pamela Michalegko, Kenneth Taylor, Benjamin Tuggle, Brian Bloodsworth,Robyn Thorson, Gary Frazer, Michael Gale, Jim Kurth, Charisa Morris,Paul Rauch, Denise Thompson, Seth Mott, Cynthia Martinez,cynthia_dohner@fws -

Association Between Systemic Inflammation and Incident Diabetes
Pathophysiology/Complications ORIGINAL ARTICLE Association Between Systemic Inflammation and Incident Diabetes in HIV-Infected Patients After Initiation of Antiretroviral Therapy 1 3 TODD T. BROWN, MD, PHD CECILIA SHIKUMA, MD nucleoside reverse transcriptase inhibi- 2 4 KATHERINE TASSIOPOULOS, DSC, MPH GRACE A. MCCOMSEY, MD tors, zidovudine and stavudine, also have 2 RONALD J. BOSCH, PHD direct and indirect effects on glucose me- tabolism (4,5). Chronic infection with HIV may also contribute to glucose ab- OBJECTIVE — To determine whether systemic inflammation after initiation of HIV- normalities among HIV-infected patients. antiretroviral therapy (ART) is associated with the development of diabetes. In the Multicenter AIDS Cohort Study, in- sulin resistance markers were higher in all RESEARCH DESIGN AND METHODS — We conducted a nested case-control study, groups of HIV-infected men compared comparing 55 previously ART-naive individuals who developed diabetes 48 weeks after ART with HIV-uninfected control subjects, initiation (case subjects) with 55 individuals who did not develop diabetes during a comparable even among those who were not receiving follow-up (control subjects), matched on baseline BMI and race/ethnicity. Stored plasma sam- ples at treatment initiation (week 0) and 1 year later (week 48) were assayed for levels of ART (6), suggesting an effect of HIV in- high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and the soluble receptors of fection itself. tumor necrosis factor-␣ (sTNFR1 and sTNFR2). Systemic inflammation has been asso- ciated with incident diabetes in multiple RESULTS — Case subjects were older than control subjects (median age 41 vs. 37 years, P ϭ cohorts in the general population (7–9). -

Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer
cancers Review Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer Viktoriia Cherkasova, Olga Kovalchuk * and Igor Kovalchuk * Department of Biological Sciences, University of Lethbridge, Lethbridge, AB T1K 7X8, Canada; [email protected] * Correspondence: [email protected] (O.K.); [email protected] (I.K.) Simple Summary: In recent years, multiple preclinical studies have shown that changes in endo- cannabinoid system signaling may have various effects on intestinal inflammation and colorectal cancer. However, not all tumors can respond to cannabinoid therapy in the same manner. Given that colorectal cancer is a heterogeneous disease with different genomic landscapes, experiments with cannabinoids should involve different molecular subtypes, emerging mutations, and various stages of the disease. We hope that this review can help researchers form a comprehensive understanding of cannabinoid interactions in colorectal cancer and intestinal bowel diseases. We believe that selecting a particular experimental model based on the disease’s genetic landscape is a crucial step in the drug discovery, which eventually may tremendously benefit patient’s treatment outcomes and bring us one step closer to individualized medicine. Abstract: Despite the multiple preventive measures and treatment options, colorectal cancer holds a significant place in the world’s disease and mortality rates. The development of novel therapy is in Citation: Cherkasova, V.; Kovalchuk, critical need, and based on recent experimental data, cannabinoids could become excellent candidates. O.; Kovalchuk, I. Cannabinoids and This review covered known experimental studies regarding the effects of cannabinoids on intestinal Endocannabinoid System Changes in inflammation and colorectal cancer. In our opinion, because colorectal cancer is a heterogeneous Intestinal Inflammation and disease with different genomic landscapes, the choice of cannabinoids for tumor prevention and Colorectal Cancer. -

Lung Anatomy
Lung anatomy Breathing Breathing is an automatic and usually subconscious process which is controlled by the brain. The brain will determine how much oxygen we require and how fast we need to breathe in order to supply our vital organs (brain, heart, kidneys, liver, stomach and bowel), as well as our muscles and joints, with enough oxygen to carry out our normal daily activities. In order for breathing to be effective we need to use our lungs, breathing muscles and blood system efficiently. This leaflet should help you to better understand the process of breathing and how we get the much needed oxygen into our bodies. The lungs You have two lungs, one in the right side and one in the left side of your chest. The right lung is bigger than the left due to the position of the heart (which is positioned in the left side of the chest). Source: Pulmonary Rehabilitation Reference No: 66354-1 Issue date: 13/2/20 Review date: 13/2/23 Page 1 of 5 Both lungs are covered by 2 thin layers of tissue called the pleura. The pleura stop the surface of the lungs rubbing together as we breathe in and out. The lungs are protected by the ribcage. The airways Within the lungs there is a vast network of airways (tubes) which help to transport the oxygen into the lungs and the carbon dioxide out. These tubes branch into smaller and smaller tubes the further they go into the lungs. Page 2 of 5 Trachea (windpipe): This tube connects your nose and mouth to your lungs. -

Innate Immunity in the Lung: How Epithelial Cells Fight Against
Copyright #ERSJournals Ltd 2004 EurRespir J 2004;23: 327– 333 EuropeanRespiratory Journal DOI: 10.1183/09031936.03.00098803 ISSN0903-1936 Printedin UK –allrights reserved REVIEW Innateimmunity in the lung: how epithelialcells ght against respiratorypathogens R. Bals*,P.S. Hiemstra # Innateimmunity in the lung: how epithelial cells ® ghtagainst respiratory pathogens *Deptof Internal Medicine, Division of R Bals, P S Hiemstra #ERS JournalsLtd 2004 PulmonaryMedicine, Hospital of the Uni- versityof Marburg, Philipps-University, ABSTRACT: Thehuman lung is exposed to a largenumber of airborne pathogens as a # resultof the daily inhalation of 10,000 litres of air Theobservation that respiratory Marburg,Germany; Deptof Pulmonology, LeidenUniversity Medical Center, Leiden, infectionsare nevertheless rare is testimony to the presence of anef®cient host defence TheNetherlands systemat the mucosal surface of the lung Theairway epithelium is strategically positioned at the interface with the Correspondence:P S Hiemstra,Dept of environment,and thus plays a keyrole in this host defence system Recognition Pulmonology,Leiden University Medical systemsemployed by airwayepithelial cells to respond to microbialexposure include the Center, P O Box9600, 2300 RC Leiden, The actionof the toll-like receptors Netherlands Theairway epithelium responds to such exposure by increasing its production of Fax:31 715266927 mediatorssuch as cytokines, chemokines and antimicrobial peptides Recent® ndings E-mail: p s hiemstra@lumc nl indicatethe importance of these peptides -

The Use of Non-Human Primates in Research in Primates Non-Human of Use The
The use of non-human primates in research The use of non-human primates in research A working group report chaired by Sir David Weatherall FRS FMedSci Report sponsored by: Academy of Medical Sciences Medical Research Council The Royal Society Wellcome Trust 10 Carlton House Terrace 20 Park Crescent 6-9 Carlton House Terrace 215 Euston Road London, SW1Y 5AH London, W1B 1AL London, SW1Y 5AG London, NW1 2BE December 2006 December Tel: +44(0)20 7969 5288 Tel: +44(0)20 7636 5422 Tel: +44(0)20 7451 2590 Tel: +44(0)20 7611 8888 Fax: +44(0)20 7969 5298 Fax: +44(0)20 7436 6179 Fax: +44(0)20 7451 2692 Fax: +44(0)20 7611 8545 Email: E-mail: E-mail: E-mail: [email protected] [email protected] [email protected] [email protected] Web: www.acmedsci.ac.uk Web: www.mrc.ac.uk Web: www.royalsoc.ac.uk Web: www.wellcome.ac.uk December 2006 The use of non-human primates in research A working group report chaired by Sir David Weatheall FRS FMedSci December 2006 Sponsors’ statement The use of non-human primates continues to be one the most contentious areas of biological and medical research. The publication of this independent report into the scientific basis for the past, current and future role of non-human primates in research is both a necessary and timely contribution to the debate. We emphasise that members of the working group have worked independently of the four sponsoring organisations. Our organisations did not provide input into the report’s content, conclusions or recommendations. -

Pneumonia: Prevention and Care at Home
FACT SHEET FOR PATIENTS AND FAMILIES Pneumonia: Prevention and Care at Home What is it? On an x-ray, pneumonia usually shows up as Pneumonia is an infection of the lungs. The infection white areas in the affected part of your lung(s). causes the small air sacs in your lungs (called alveoli) to swell and fill up with fluid or pus. This makes it harder for you to breathe, and usually causes coughing and other symptoms that sap your energy and appetite. How common and serious is it? Pneumonia is fairly common in the United States, affecting about 4 million people a year. Although for many people infection can be mild, about 1 out of every 5 people with pneumonia needs to be in the heart hospital. Pneumonia is most serious in these people: • Young children (ages 2 years and younger) • Older adults (ages 65 and older) • People with chronic illnesses such as diabetes What are the symptoms? and heart disease Pneumonia symptoms range in severity, and often • People with lung diseases such as asthma, mimic the symptoms of a bad cold or the flu: cystic fibrosis, or emphysema • Fatigue (feeling tired and weak) • People with weakened immune systems • Cough, without or without mucus • Smokers and heavy drinkers • Fever over 100ºF or 37.8ºC If you’ve been diagnosed with pneumonia, you should • Chills, sweats, or body aches take it seriously and follow your doctor’s advice. If your • Shortness of breath doctor decides you need to be in the hospital, you will receive more information on what to expect with • Chest pain or pain with breathing hospital care. -

Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases
microorganisms Review Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases Juan Alberto Lira-Lucio 1 , Ramcés Falfán-Valencia 1 , Alejandra Ramírez-Venegas 2, Ivette Buendía-Roldán 3 , Jorge Rojas-Serrano 4 , Mayra Mejía 4 and Gloria Pérez-Rubio 1,* 1 HLA Laboratory, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] (J.A.L.-L.); [email protected] (R.F.-V.) 2 Tobacco Smoking and COPD Research Department, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] 3 Translational Research Laboratory on Aging and Pulmonary Fibrosis, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] 4 Interstitial Lung Disease and Rheumatology Unit, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] (J.R.-S.); [email protected] (M.M.) * Correspondence: [email protected]; Tel.: +52-55-5487-1700 (ext. 5152) Received: 11 June 2020; Accepted: 7 July 2020; Published: 16 July 2020 Abstract: The lung microbiome composition has critical implications in the regulation of innate and adaptive immune responses. Next-generation sequencing techniques have revolutionized the understanding of pulmonary physiology and pathology. Currently, it is clear that the lung is not a sterile place; therefore, the investigation of the participation of the pulmonary microbiome in the presentation, severity, and prognosis of multiple pathologies, such as asthma, chronic obstructive pulmonary disease, and interstitial lung diseases, contributes to a better understanding of the pathophysiology. Dysregulation of microbiota components in the microbiome–host interaction is associated with multiple lung pathologies, severity, and prognosis, making microbiome study a useful tool for the identification of potential therapeutic strategies. -
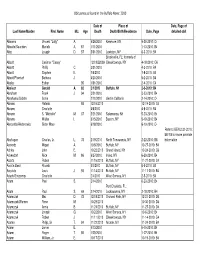
Obituaries Buffalo News 2010 by Name
Obituaries as found in the Buffalo News: 2010 Date of Place of Date, Page of Last Name/Maiden First Name M.I. Age Death Death/Birth/Residence Date, Page detailed obit Abbarno Vincent "Lolly" A. 9/26/2010 Kenmore, NY 9-30-2010: C4 Abbatte/Saunders Murielle A. 87 1/11/2010 1-13-2010: B4 Abbo Joseph D. 57 5/31/2010 Lewiston, NY 6-3-2010: B4 Brooksville, FL; formerly of Abbott Casimer "Casey" 12/19/22009 Cheektowaga, NY 4-18-2010: C6 Abbott Phillip C. 3/31/2010 4-3-2010: B4 Abbott Stephen E. 7/6/2010 7-8-2010: B4 Abbott/Pfoetsch Barbara J. 4/20/2010 5-2-2010: B4 Abeles Esther 95 1/31/2010 2-4-2010: C4 Abelson Gerald A. 82 2/1/2010 Buffalo, NY 2-3-2010: B4 Abraham Frank J. 94 3/21/2010 3-23-2010: B4 Abrahams/Gichtin Sonia 2/10/2010 died in California 2-14-2010: C4 Abramo Rafeala 93 12/16/2010 12-19-2010: C4 Abrams Charlotte 4/6/2010 4-8-2010: B4 Abrams S. "Michelle" M. 37 5/21/2010 Salamanca, NY 5-23-2010: B4 Abrams Walter I. 5/15/2010 Basom, NY 5-19-2010: B4 Abrosette/Aksterowicz Sister Mary 6/18/2010 6-19-2010: C4 Refer to BEN 2-21-2010: B6/7/8 for more possible Abshagen Charles, Jr. L. 73 2/19/2010 North Tonawanda, NY 2-22-2010: B8 information Acevedo Miguel A. 10/6/2010 Buffalo, NY 10-27-2010: B4 Achkar John E.