Thermal Decomposition Kinetics of Kerosene-Based Rocket Propellants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polymerization of Safflower Oil in a Diesel Lubrication System by John Walter Olson a Thesis Submitted in Partial Fulfillment Of
Polymerization of safflower oil in a diesel lubrication system by John Walter Olson A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by John Walter Olson (1988) Abstract: Oxidative addition polymerization of lubrication oil contaminated with safflower oil diesel fuel was investigated in laboratory apparatus simulating conditions prevailing in a diesel engine crankcase. Primary research objectives were the identification and testing of key additives which might suppress problems of excessive viscosity rise and alkalinity decline of lubrication/safflower oil mixtures. Both commercial and base stock medium-speed locomotive lubrication oils were investigated with 5.0 weight percent safflower oil contamination. Oil mixtures were exposed to elevated temperature and were contacted by percolation with either oxygen or nitrogen to provide an oxidizing atmosphere and agitation or simply agitation. Copper, a known diesel engine wear metal, was introduced as a polymerization catalyst. Lubrication oils were evaluated with and without safflower oil and additives. Both commercial and base stock oils with no safflower oil were found to polymerize at a moderate baseline level. Amines were found to show little alkalinity maintenance efficacy while performance by an overbased calcium phenate was relatively good. Numerous antioxidants were tested for ability to disrupt the mechanisms leading to polymerization. Two widely-used antioxidants, zinc dialkyldithiophosphate and zinc diamyl-dithiocarbamate, were found to promote polymerization of oil mixtures in this research. This unexpected result is almost certainly a result of promotion catalysis by zinc. A metal deactivator N-N'-disalicylidene-1,2-propanediamine retarded viscosity rise, apparently by chelating wear metal catalysts. -

(Copcs) Recommendations Fuel Additives Red Hill Bulk Fuel Storage Fuel Facility
Chemicals of Potential Concern (COPCs) Recommendations Fuel Additives Red Hill Bulk Fuel Storage Fuel Facility A meeting was held on May 10, 2016 to discuss the recommended approach to addressing the objectives of the Administrative Order on Consent (AOC) In the Matter of Red Hill Bulk Fuel Storage Facility (herein referred to as “the Facility”) Statement of Work (SOW) Section 6 and Section 7 with the Regulatory Agencies (State of Hawaii Department of Health [DOH] and United States Environmental Protection Agency Region IX [EPA]) and various subject matter experts (SMEs). The following attended the meeting: Parties of the AOC (Regulatory Agencies, Department of Navy [Navy], and Defense Logistics Agency [DLA]) and SMEs to the Regulatory Agencies (University of Hawaii [UH]; State of Hawaii Department of Land and Natural Resources [DLNR] Commission on Water Resources Management [COWRM]; United States Geological Survey [USGS] Pacific Islands Water Science Center; City and County of Honolulu Board of Water Supply [BWS]). Also in attendance were the Navy’s contractor, AECOM, and BWS’ contractor, Intera Geoscience & Engineering Solutions (Intera). One of the action items from the meeting was for the Navy and DLA to evaluate fuel additives and determine if additional analytes need to be included on the chemicals of potential concern (COPCs) list for the Facility, as previously agreed upon by the Parties of the AOC on February 4, 2016. The following discussion and table present the results of the fuel additives evaluation: Table 1 summarizes 18 chemical constituents of additives associated with fuel stored at the Facility. Six groups of fuel additives were identified and evaluated: (1) metal deactivators; (2) corrosion inhibitors and lubricity improvers; (3) icing inhibitors; (4) static dissipaters; (5) lubricity improvers; and (6) antioxidants. -
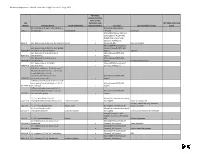
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

Product Specifications Aviation Kerosene JP8
Product Specifications Aviation Kerosene JP8 TEST METHODS (1) PROPERTY UNITS LIMITS ASTM IP STANDARDS STANDARDS Clear, bright and visually free from solid Appearance matter and undissolved water at Visual ambient temperature. In case of dispute it shall be evaluated at 21º C. standard D 156 (2) Saybolt color inform scale D 6045 Total acidity mg KOH/g maximum 0,015 D 3242 IP 354 Aromatics % V/V maximum 25,0 D 1319 (2) IP 156 maximum 26,5 D 6379 IP 436 Sulfur, Total % m/m maximum 0,30 D 129 IP 336 D 1266 D 2622 D 3120 (3) D 4294 (2) D 5453 Sulfur, Mercaptan or % m/m maximum 0,002 D 3227 (2) IP 342 Doctor Test (4) Doctor Negative D 4952 Distillation (5): D 86 (2) (6) IP 123 Initial boiling point report D 2887 IP 406 ºC 10 % V/V recovered maximum 205 D 7345 (7) ºC 20 % V/V recovered report ºC 50 % V/V recovered report ºC 90 % V/V recovered report ºC End point maximum 300 % V/V Residue maximum 1,5 % V/V Loss maximum 1,5 Flash point (8) ºC minimum 38 D 56 IP 170 D 93 (2) D 3828 Density at 15 ºC or kg/m³ 775 a 840 D 1298 IP 160 Density ºAPI at 60 ºF º API 37,0 a 51,0 D 4052 (2) IP 365 D 7777 Freezing point ºC maximum -47 D 2386 (2) IP 16 D 5972 IP 435 D 7153 IP 529 D 7154 IP 528 Viscosity at -20 ºC mm²/s maximum 8.0 D 445 (2) IP 71 Section 1 D 7042 (9) D 7945 Specific energy MJ/kg minimum 42.8 D 3338 IP 12 D 4529 D 4809 (2) Hydrogen content % m/m minimum 13,4 D 3343 D 3701 D 5291 D7171 (2) Smoke point or mm minimum 25,0 D 1322 IP 598 Smoke point and mm minimum 19.0 D 1322 IP 598 Naphthalenes % V/V maximum 3.0 D 1840 Calculated cetane index report D 976 D 4737 Copper corrosion, 2 h at 100 ºC (212 ºF) Class maximum 1b D 130 IP 154 MIL-DTL-83133K 11, July 18, 2018 Page 1of 5 TEST METHODS (1) PROPERTY UNITS LIMITS ASTM IP STANDARDS STANDARDS Thermal stability (JFTOT) (15): D 3241 IP 323 Test temperature tube rating ºC minimum 260 Tube evaluation, one of the following requirements shall be met (16) (17): maximum <3. -

Lubricant Additives Lubricant Additives
Worldwide Ltd LUBRICANT ADDITIVES LUBRICANT ADDITIVES VANDERBILT WORLDWIDE Ltd 12 Park House Alvaston Business Park, Middlewich Rd. Nantwich, Cheshire CW5 6PF, UK +44 1270 623978 [email protected] www.vanderbiltworldwide.com VANDERBILT CHEMICALS, LLC CERTIFIED TO ISO 9001:2015 10002461 NSF® Certified Registered and pending trademarks appearing in these materials are those of R.T. Vanderbilt Holding Company, Inc. or its respective wholly owned subsidiaries. For complete listings, please visit this location for trademarks, www.rtvanderbiltholding.com. NSF is a registered trademark of NSF International. UL is a registered trademark of UL LLC. TPS is a registered trademark of Arkema France Corporation Rev. 01/06/2020 DISCLAIMER Before using, read, understand and comply with the information and precautions in the Safety Data Sheets, label and other product literature. The information presented herein, while not guaranteed, was prepared by technical personnel and, to the best of our knowledge and belief, is true and accurate as of the date hereof. No warranty, representation or guarantee, express or implied, is made regarding accuracy, performance, stability, reliability or use. This information is not intended to be all-inclusive, because the manner and conditions of use, handling, storage and other factors may involve other or additional safety or performance considerations. The user is responsible for determining the suitability of any material for a specific purpose and for adopting such safety precautions as may be required. Vanderbilt Chemicals, LLC does not warrant the results to be obtained in using any material, and disclaims all liability with respect to the use, handling or further processing of any such material. -

IIIHHHHHHHHHHH USOO5298.178A United States Patent (19) 11 Patent Number: 5,298,178 O'neil Et Al
IIIHHHHHHHHHHH USOO5298.178A United States Patent (19) 11 Patent Number: 5,298,178 O'Neil et al. 45) Date of Patent: Mar. 29, 1994 (54) TRIAZOLE COMPOUNDS USEFUL AS 4,701,273 10/1987 Brady ................................. 252/32.5 4,791,206 12/1988 O'Neil et al. ... 548/108 METAL DEACTIVATORS 4,855,070 8/1989 Lewis .................................... 252/77 75) Inventors: Robert M. O'Neil, Flixton, England; 4,886,611 12/1989 Kapusinski et al. .................. 252/47 Paul Dubs, Marly, Switzerland 4,897,086 l/1990 Blair et al. 4,944,616 8/1990 Falla et al. ............................ 252/77 73) Assignee: Ciba-Geigy Corporation, Ardsley, 4,992, 185 2/1991 Shim ................. ... 252/77 N.Y. 5,021,181 6/1991 Kanamori et al. .................... 252/77 5,032,300 7/1991 O'Neil ...................... 252/.51.5 R (21) Appl. No.: 879,484 5,076,946 12/1991 Frankenfeld et al. ".co- 548/257 22 Filed: May 7, 1992 FOREIGN PATENT DOCUMENTS Related U.S. Application Data 0006710 1/1980 European Pat. Off... 62) Division of Ser. No. 713,943, Jun. 12, 1991, Pat. No. Primary Examiner-Margaret Medley 5,133,890. Attorney, Agent, or Firm-Luther A. R. Hall (30) Foreign Application Priority Data (57) ABSTRACT Jun. 13, 1990 (GB) United Kingdom ................. 9013142 Composition comprising an aqueous or partially aque ous functional fluid susceptible to degradation by a 51) int. Cl. ................ C10M 173/00; C10M 173/02; COM 33/44 metal; and b) a metal deactivator having the formula I: 52 U.S. Cl. .............................. 252/49.3; 252/51.5 R; 252/77 (58) Field of Search ..................... -

Used Oil Re-Refining Study to Address Energy Policy Act of 2005 Section 1838
U.S. Department of Energy • Offi ce of Fossil Energy Used Oil Re-refi ning Study to Address Energy Policy Act of 2005 Section 1838 July 2006 Used Oil Re-refining Study to Address Energy Policy Act of 2005 Section 1838 Office of Oil and Natural Gas Office of Fossil Energy U.S. Department of Energy Used Oil Re-refining Study to Address Energy Policy Act of 2005 Section 1838 Table of Contents 1 Executive Summary........................................................................................... 1-1 1.1 Methodology.............................................................................................. 1-1 1.2 Executive Summary Roadmap.................................................................. 1-1 1.3 Worldwide Lube Oil Demand Perspective and Used Oil Management Practices.................................................................................................... 1-2 1.4 Stakeholders in the U.S. Used Oil Business ............................................. 1-3 1.5 Energy and Environmental Impacts........................................................... 1-4 1.6 Existing Federal and State Regulations Dealing with Used Oils ............... 1-5 1.7 Reusing a Valuable Resource................................................................... 1-6 1.8 Specific Steps That Could be Taken to Improve Collections and Increase Re-refining and Other Beneficial Re-use of Used Oils ............... 1-7 1.8.1 Utilize Information Exchange Processes to Encourage Progress on Used Oil Management and Recycling ................................................. -

Hitec® 10400U Corrosion Inhibitor & Lubricity Improver
FUELS HiTEC® 10400U Corrosion Inhibitor & Lubricity Improver Multifunctional Additive that Minimizes Both Color Degradation and Sediment Formation FUELS HiTEC® 10400U Corrosion Inhibitor & Lubricity Improver Multifunctional Additive that Minimizes Both Color Degradation and Sediment Formation Application Copper Deactivation HiTEC® 10400U corrosion inhibitor and lubricity improver is a complex Test: Diesel Fuel Heated 7 Days @ 79°C (175°F) metal deactivator which is effective on iron, copper, and several other Added ASTM Sediment metals. HiTEC® 10400U not only deactivates the deleterious trace metal Metal Deactivator ppm Cu ppm D1500 Color mg/100 ml contaminants, but it also inhibits oxidation and esterification reactions None 0 0 2.5 4.2 in the absence of these metal species. HiTEC® 10400U will provide the required stability as a single chemical treatment on most fuels. The None 0 1 3 5.6 required stability is normally specified by the refining company, pipeline Competitive 26 1 3.5 2.8 company, or end user. HiTEC® 10400U is a multifunctional additive that HiTEC® 10400U 21 1 2.5 2.0 minimizes both color degradation and sediment formation. HiTEC® 10400U will effectively deactivate metals in home heating oil, Typical Characteristics gasoline, diesel fuels, turbine fuels, kerosene, and residual fuel oils. It is Appearance Amber red brown clear liquid effective on both virgin and cracked distillates. It can be employed as a Specific Gravity @ 77°F/25°C 0.928 single chemical treatment or it can be used to enhance the performance Flash Point, °C (C) 70 min. of other stability additives. Stability testing on a candidate fuel will indicate the fuel's specific requirement. -

Fuel Additives Product Summary Fuel Additives Product Summary 3
For more information on how Innospec’s Fuel Specialties team can work with you, please contact our regional sales office. Our international office network offers unrivalled product support and it has the resources to deliver the required fuel additive treatments to customers anywhere in the world. Innospec Fuel Specialties LLC 8375 South Willow Street Littleton, Colorado 80124 Tel: 303-792-5554 Netherlands Denmark Norway Sweden Finland Belgium Belarus Canada Yugoslavia UK Edmonton Germany Bulgaria Hungary Montreal Czech Republic Ukraine Indiana Greece Colorado Illinois France Korea Spain Italy Turkey Cyprus California Pennsylvania Lebanon USA Syria Japan Texas China South Iraq Kuwait Carolina Morocco Switzerland Tunisia Iran Alabama Pakistan Egypt Bahrain Bangladesh Algeria India Monterrey Jordan Senegal Dubai Hong Kong Benin Togo Sudan Vietnam Mexico City Nigeria Yemen Philippines United Arab Emirates (UAE) Thailand Venezuela Burkina Faso Ethiopia Costa Rica Saudi Arabia Singapore Malaysia Columbia Uganda Ghana Sri Lanka Cameroon Congo Ecuador Ivory Coast Kenya Gabon Tanzania Peru Tahiti Indonesia Bolivia Brazil Reunion Chile Sydney Uruguay Melbourne New Zealand Argentina South Africa Fuel Additives Innospec Limited Innospec Limited Innospec Manufacturing Park Branch Office Singapore Product Summary Oil Sites Road, Ellesmere Port 47 Scotts Road Cheshire, CH65 4EY, UK #02-03 Goldbell Towers Tel: +44 (0)151 355 3611 Singapore 228233 Tel: +65-6336 6286 www.innospecinc.com 2 Fuel Additives Product Summary Fuel Additives Product Summary 3 Refinery Specialties Fuel Antioxidants The purpose of an antioxidant additive AO-22: N,N’-di-sec-butyl-p-phenylenediamine. Readily soluble in gasoline in all proportions. Sp. Gr. 0.94. is to extend the induction period and USE: Sweetens gasoline and retards formation of gum. -

Dee-Zol Life® Vs. the Marketplace Introduction Diesel Fuel Stabilizers
Bell Performance, Inc. tel 407-831-5021 1340 Bennett Drive fax 407-331-1125 Longwood, FL 32750 www.bellperformance.com www.WeFixFuel.com Product Comparison Files: Dee-Zol Life® vs. The Marketplace Bell Performance invented the first fuel additive in the year 1909 and has been formulating effective problem-solving fuel treatments ever since. In response to the market’s widespread need for petroleum fuel stabilizers and anti-oxidants, Bell Performance formulated Dee-Zol Life® in 2007 as a dedicated solution for fuel users who store diesel fuel long-term for various applications. In this report, we’ll compare the important functions and features of diesel fuel stabilizers and see how some of the prominent options in the marketplace compare with Dee-Zol Life®. Introduction Today’s petroleum fuels (diesel and gasoline) have a greater need for fuel stabilizers than ever before. This is due to changes in chemical makeup resulting from extensive refinery cracking and processing of these fuels. Today’s stored fuels have significantly shorter storage lives – as little of 20% of what you could have expected from comparable fuels a few decades ago. Diesel Fuel Stabilizers - What They’re Used For Petroleum fuels have always been susceptible to chemical breakdown as they are exposed to environmental actors. Exposure to oxygen, water, light, heat and catalytic metals will accelerate chemical reactions involving unstable precursors that exist in the fuel from the start of its life. Left unchecked, this phenomenon will shorten the effective storage life of petroleum fuels. Unstable fuel has signature hallmarks – darkening of color, stratification of light and dark phases within the fuel, and formation of gums, resins and sludge. -

Aviation Fuels Technical Review
Aviation Fuels Technical Review | Chevron Products Aviation Fuels Company Technical Review Chevron Products Company 6001 Bollinger Canyon Road San Ramon, CA 94583 Chevron Products Company is a division of a wholly owned subsidiary of Chevron Corporation. http://www.chevron.com/productsservices/aviation/ © 2007 Chevron U.S.A. Inc. All rights reserved. Chevron and the Caltex, Chevron and Texaco hallmarks are federally registered trademarks of Chevron Intellectual Property LLC. Recycled/RecyclableRecycled/recyclable paper paper IDC 1114-099612 MS-9891 (11/14) Table of Contents Notes General Introduction ............................................................i 8 • Aviation Gasoline Performance ............................ 45 Performance Properties 1 • Aviation Turbine Fuel Introduction ........................... 1 Cleanliness Types of Fuel Safety Properties Fuel Consumption 9 • Aviation Gasoline 2 • Aviation Turbine Fuel Performance .........................3 Specifications and Test Methods .......................... 54 Performance Properties Specifications Cleanliness Future Fuels Safety Properties Test Methods Emissions 10 • Aviation Gasoline Composition ............................. 63 3 • Aviation Turbine Fuel Composition Specifications and Test Method ...............................14 Property/Composition Relationships Specifications Additives Test Methods 11 • Aviation Gasoline Refining ..................................... 66 4 • Aviation Turbine Fuel Composition ........................24 Alkylation Base Fuel Avgas Blending -

OMEGA 909 SUPER ACTING ADDITIVES: Many of the Better Engine Oils Include Three Or Four Useful Additives
909 SUPER ENGINE OIL ADDITIVE: sounding terms to describe what are basically Probably the most important, yet least detergent-type additives or heavy oil additives. understood ingredient of most modern engine Although the phosphorus, sulfur and chlorine oils are the additives that are included in the oil containing additives do keep crankcases free of itself. Most additives fall into one of two main gumming and carbon deposits through their categories. One category of detergent-type detergent action, during their breakdown additives contains phosphorus, sulfur or immediately afterwards, they form highly chlorine derivatives and the other contains corrosive elements that in turn attack the proportions of heavy oil. Omega 909 is unique internal engine part metals, causing premature in that it does not fall into either category but failure of pistons, valves and rings. instead uses a completely new and advanced chemical action that is superior to both. The second category of engine oil additives is nothing more than the addition of a heavier ORDINARY OIL ADDITIVES: grade of low viscosity oil, which is included in At present, the major oil companies market their the belief that 'a heavy oil in the engine oil is a engine oils under a multitude of types but all of good thing'. However, no scientific proof exists them fall into one of the two above categories. to substantiate such an unscientific second Their marketing people make up scientific guess. OMEGA 909 SUPER ACTING ADDITIVES: Many of the better engine oils include three or four useful additives. Omega 909 super engine oil additive contains twenty-two.