Blueprint for an Intestinal Villus: Species-Specific Assembly Required
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcytosis of Listeria Monocytogenes Across The
Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, Marc Lecuit To cite this version: Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, et al.. Tran- scytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. Journal of Experimental Medicine, Rockefeller University Press, 2011, 208 (11), pp.2263-2277. 10.1084/jem.20110560. pasteur-02040395 HAL Id: pasteur-02040395 https://hal-pasteur.archives-ouvertes.fr/pasteur-02040395 Submitted on 20 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Published Online: 3 October, 2011 | Supp Info: http://doi.org/10.1084/jem.20110560 Downloaded from jem.rupress.org on February 19, 2019 Article Transcytosis of Listeria monocytogenes across -

Regulation of Intestinal Blood Flow
Journal of Surgical Research 93, 182–196 (2000) doi:10.1006/jsre.2000.5862, available online at http://www.idealibrary.com on RESEARCH REVIEW Regulation of Intestinal Blood Flow Paul J. Matheson, Ph.D.,*,†,1 Mark A. Wilson, M.D., Ph.D.,*,†,‡ and R. Neal Garrison, M.D.*,†,‡ *Center for Excellence in Applied Microcirculatory Research and ‡Department of Surgery, University of Louisville, Louisville, Kentucky 40292; and †Louisville Veterans Affairs Medical Center, Louisville, Kentucky 40206 Submitted for publication July 29, 1999 arteries is typically 20–25% of cardiac output in the The gastrointestinal system anatomically is posi- unfed state [2]. There is extensive overlap or collateral tioned to perform two distinct functions: to digest and circulation in the distal vascular distributions of these absorb ingested nutrients and to sustain barrier func- arteries. During nutrient absorption, blood flow in each tion to prevent transepithelial migration of bacteria of these arteries is increased sequentially as the diges- and antigens. Alterations in these basic functions con- tive chyme passes over the mucosal surface supplied by tribute to a variety of clinical scenarios. These pri- mary functions intrinsically require splanchnic blood the particular arteries [3]. Following nutrient absorp- flow at both the macrovascular and microvascular lev- tion, the blood flow to each segment returns to baseline els of perfusion. Therefore, a greater understanding of levels as the chyme moves past that region of the the mechanisms that regulate intestinal vascular per- digestive tract [4, 5]. This postprandial increase in fusion in the normal state and during pathophysiolog- blood flow is independent of organ distention and is ical conditions would be beneficial. -
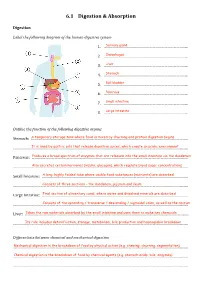
Topic 6.1 Answers
6.1 Digestion & Absorption Digestion Label the following diagram of the human digestive system 1. ………………………………………………………………………...Salivary gland 2. ………………………………………………………………………...Oesophagus 3. ………………………………………………………………………...Liver 4. ………………………………………………………………………...Stomach 5. ………………………………………………………………………...Gall bladder 6. ……Pancreas…………………………………………………………………... 7. ………………………………………………………………………...Small intestine 8. ………………………………………………………………………...Large intestine Outline the function of the following digestive organs Stomach: ………………………………………………………………………………………A temporary storage tank where food is mixed by churning and………………………………………………… protein digestion begins …………………………………………………………………………………………………………………………………………………..........It is lined by gastric pits that release digestive juices, which create an acidic environment Pancreas: …………..……………………………………………………………………………………………………………………………Produces a broad spectrum of enzymes that are released into the small intestine via the duodenum …………………………………………………………………………………………………………………………………………………..........Also secretes certain hormones (insulin, glucagon), which regulate blood sugar concentrations Small Intestine: …………….……………………………………………………………………………………………A long, highly folded tube where usable food substances (nutrients) are absorbed…………………… …………………………………………………………………………………………………………………………………………………..........Consists of three sections – the duodenum, jejunum and ileum Large Intestine: …………….…………………………………………………………………………………………………………………Final section of alimentary canal, where water and dissolved minerals are absorbed …………………………………………………………………………………………………………………………………………………..........Consists -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

The Number of Villi in Rat's Jejunum and Ileum: Effect of Normal Growth, Partial Enterectomy, and Tube Feeding
J. Anat. (1972). 111, 2, pp. 283-291 283 With 6 figures Printed in Great Britain The number of villi in rat's jejunum and ileum: effect of normal growth, partial enterectomy, and tube feeding J. M. FORRESTER Department of Physiology, Edinburgh University (Accepted 8 January 1972) INTRODUCTION The villi of the rat small intestine are covered by enterocytes which have a life-span, from their time of origin in the crypts until shedding at the villus tip, of only about one and a half days (Leblond & Stevens, 1948; Bertalanffy, 1960). Their shape varies from one part of the small intestine to another, and even adjacent villi may differ strikingly. In view of these features suggesting a rapidly changing scene, this paper describes a procedure for enumerating the villi in rat jejunum and ileum, and ex- amines the stability of the total number during normal growth, after partial enter- ectomy, and after tube feeding. METHODS Locally bred Wistar male rats were used. They were fed on a standard pelleted rat food manufactured in Edinburgh. They always had tap water ad libitum. Enumeration procedure. Rats were killed by inhalation of chloroform in the morn- ing. The position of the suspensory ligament was marked on the small intestine where a band of connective tissue is attached to the intestine at the duodenojejunal junction. Then the small intestine was removed from pylorus to ileocolic valve by gentle traction, and washed through with cold saline (NaCl 0-9 %, w/v). It was weighed, and after removal of the duodenum, was laid in a trough and perfused with Bouin's solution at an outlet pressure of 20 cm of solution for at least 20 minutes (Hromadkova & Skala, 1969). -

An Electron Microscopic Study of the Intestinal Villus I
An Electron Microscopic Study of the Intestinal Villus I. The Fasting Animal By SANFORD L. PALAY,* M.D., and LEONARD J. KARLIN, M.I). (From the Department of Anatomy, Yale University School of Medicine, New Haven, and the Laboratory of Neuroanalomical Sciences, National Institute of Neurological Diseases and Blindness, National Institutes of Health, Bethesda) PLATES 148 TO 159 (Received for publication, January 16, 1959) ABSTRACT The structure of the intestinal villus of the rat was studied in thin sections of tissue fixed in buffered osmium tetroxide and embedded in methacrylate. The simple columnar epithelium investing the villus is surmounted by a striated border consisting of slender projections of the cell surface. These microvilli are arranged in almost crystalline, hexagonal array, and increase the apical surface area of the cell by a factor of 24. The core of each microvillus is filled with fine fibrils which arise from the filamentous substance of the terminal web underlying the striated border. Each microvillus is covered by a tubular extension of the plasma membrane of the epithelial cell. Pinocytotic vesicles originating from the plasma membrane occur at the bases of the intermicrovillous spaces. The nucleus, mitochondria, and the endoplasmic reticuIum of the epithelial cell display no unusual features. Small bits of ergastoplasm occur in the apical cytoplasm. A thin basement membrane separates the epithelium from the lamina propria which consists of vessels, nerves, and numerous lymphocytes, eosinophiles, mast cells, plasma cells, smooth muscle fibers, and macrophages suspended in a delicate stroma of fibroblasts and collagen fibers. Intercellular fat droplets often occur in this stroma, even in animals fasted for 40 hours. -
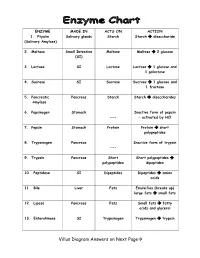
Villus Diagram Answers on Next Page→
ENZYME MADE IN ACTS ON ACTION 1. Ptyalin Salivary glands Starch Starch disaccharide (Salivary Amylase) 2. Maltase Small Intestine Maltose Maltose 2 glucose (SI) 3. Lactase SI Lactose Lactose 1 glucose and 1 galactose 4. Sucrase SI Sucrose Sucrose 1 glucose and 1 fructose 5. Pancreatic Pancreas Starch Starch disaccharides Amylase 6. Pepsinogen Stomach Inactive form of pepsin --- – activated by HCl 7. Pepsin Stomach Protein Protein short polypeptides 8. Trypsinogen Pancreas Inactive form of trypsin --- 9. Trypsin Pancreas Short Short polypeptides polypeptides dipeptides 10. Peptidase SI Dipeptides Dipeptides amino acids 11. Bile Liver Fats Emulsifies (breaks up) large fats small fats 12. Lipase Pancreas Fats Small fats fatty acids and glycerol 13. Enterokinase SI Trypsinogen Trypsinogen trypsin Villus Diagram Answers on Next Page Intestinal villus READ INFORMATION BELOW!!! From Wikipedia, the free encyclopedia Intestinal villi (singular: villus) are tiny, finger-like projections that protrude from the epithelial lining of the intestinal wall. Each villus is approximately 0.5-1.6 mm (millimetres) in length and has many microvilli (singular: microvillus), each of which are much smaller than a single villus. Intestinal villi should not be confused with the larger folds of mucous membrane in the bowel known as the plicae circulares. A villus is much smaller than a single fold of plicae circulares. Villi increase the internal surface area of the intestinal wall. Increased surface area allows for increased intestinal wall area that is available for absorption. Increased absorptive area is useful because digested nutrients (including sugars and amino acids) pass into the villi which is semi permeable, through diffusion, which is effective only at short distances. -

Histomorphometric Evaluation of Small Intestinal Mucosa of Red Jungle Fowl and Commercial Broiler from One Day to Four Months of Age
African Journal of Biotechnology Vol. 11(7), pp. 1806-1811, 24 January, 2012 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.664 ISSN 1684–5315 © 2012 Academic Journals Full Length Research Paper Histomorphometric evaluation of small intestinal mucosa of red jungle fowl and commercial broiler from one day to four months of age K. Khalid Kadhim 1, A. B. Z. Zuki 2*, M. M. Noordin 3, S. M. A. Babjee 3 and M. Zamri-Saad 3 1Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor. 2Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor. 3Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor. Accepted 25 May, 2011 Histomorphometry of the small intestinal mucosa of the red jungle fowl (RJF) and commercial broiler breed (CBC) from day one to four months post-hatch were investigated. For the sake of comparison between these two breeds, the following parameters were included: the number of villi, villus surface area and the intestinal surface area for each small intestinal segment. New procedure for enumerating the intestinal villi was performed by using the standard paraffin sections, whereby the villi were counted through their cross sections using an image analyzer. The numbers of villi were significantly higher in the RJF than the CBC for all the intestinal segments from the day one to four months post- hatch. The villi numbers showed a decreased with age. The villus surface area was significantly higher in the CBC than the RJF for all intestinal segments for all the ages. -

Morphology of Small Intestinal Mucosa and Intestinal Weight Change with Metabolic Type of Cattle
Veterinarni Medicina, 53, 2008 (10): 525–532 Original Paper Morphology of small intestinal mucosa and intestinal weight change with metabolic type of cattle R. Zitnan1,3, J. Voigt1, S. Kuhla1, J. Wegner2, A. Chudy1, U. Schoenhusen1, M. Brna3, M. Zupcanova3, H. Hagemeister1 1Research Unit Nutritional Physiology “Oskar Kellner”, 2Research Unit Muscle Biology & Growth, Research Institute for the Biology of Farm Animals, Dummerstorf, Germany 3Slovak Agricultural Research Centre, Research Institute of Animal Production, Department of Animal Nutrition, Nitra, Slovak Republic ABSTRACT: The objective of this study was to investigate rumen fermentation, apparent digestibility of nutri- ents, and morphology of ruminal und intestinal mucosa in two cattle breeds of different metabolic type. From each breed six purebred German Holstein (H) bulls representing the secretion type and six Charolais (CH) bulls representing the accretion type were raised and fattened under identical conditions with semi ad libitum feeding of a high energy diet. The animals were used for a digestion trial started at nine months of age and animals were slaughtered at 18 months of age. Body weight (668 vs. 764 kg, P = 0.011), body weight gain (1 223 vs. 1 385 g/day, P = 0.043), and body protein gain (93 vs. 128 g/day, P = 0.001) were lower in H compared to CH bulls. Protein expense per kg protein accretion was higher in H bulls (13.8 vs. 10.2, P = 0.001). No significant differences were found in concentration and pattern of ruminal short chain fatty acid and in apparent digestibility of organic matter, crude fibre, and N-free extracts. -

The Digestive, Reproductive, and Urinary Systems
The Digestive, Reproductive, and Urinary Systems THE DIGESTIVE SYSTEM Your body is constantly using energy, even when you’re at rest. Your cells use energy to carry out the normal functions of protein synthesis, cell maintenance and repair, and their own particular functions. On a larger scale, processes such as breathing, pumping of the heart, maintenance of normal levels of substances within the body, and digestion and absorption of foods are vital to life. All these processes continue while you’re sleeping. Because your body can’t man- ufacture energy, it must obtain that energy from elsewhere. In all animals, energy comes from food. Food also provides the body with fresh raw materials for growth, maintenance, and repair of body structures. The digestive system deals with the intake, physical breakdown, chemical digestion, and absorption of food, along with the elimination of waste products created by this process. The digestive system also eliminates certain toxic substances and secretes hormones it uses to regulate itself. The Oral Cavity The mouth, or oral cavity, consists of the lips, teeth and gums, tongue, oropharynx, and the associated salivary glands. The lips are a zone of transition from the skin of the face to the mucous membrane (a general term denoting the surface of an organ lubricated by moisture) lining the gums and the inside of your cheeks. Several layers of muscle help the lips grab and retain food and water within the mouth. 1 Different animals have different degrees of lip muscle devel- opment. Grazing animals like cattle, sheep, and horses have muscular lips that are prehensile (i.e., adapted to grasp plant material). -

Morphological Development of the Small Intestine in White Roman Goslings
African Journal of Biotechnology Vol. 12(6), pp. 611-617, 6 February, 2013 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.1526 ISSN 1684–5315 ©2013 Academic Journals Full Length Research Paper Morphological development of the small intestine in white Roman goslings Bor-Ling SHIH1,2, Yieng-How CHEN3 and Jenn-Chung HSU1* 1Department of Animal Science, National Chung Hsing University, 250 Kao-Kung Road, Taichung 402, Taiwan, Republic of China. 2Nutrition Division, Livestock Research Institute, Council of Agriculture, Hsinhua, Tainan 712, Taiwan, Republic of China. 3Department of Animal Science, Tung-Hai University, 181 Taichung Harbor Road, Section 3 Taichung 406, Taiwan, Republic of China. Accepted 21 May, 2012 The objective of this study was to investigate the morphological development of small intestinal segments in white Roman goslings from hatching to 28 days of age. Forty day-old male goslings were used in this experiment. Eight goslings were selected randomly at hatching, 7, 14, 21 and 28 days of age, respectively. The small intestinal segments were sampled from six goslings for intestinal structure observation by light microscopy and from two male goslings for scanning electron microscopy (SEM). The villus height, width, perimeter, area, crypt depth, muscle thickness and height/width ratio significantly (P < 0.05) increased during the first four weeks post-hatching. The villus width of duodenum continued to increase (P < 0.05) until 28 days of age, whereas the jejunum and ileum villus width reached a peak at 21 days of age. The development of villus height, area and crypt depth increased two to three times in the small intestine of goslings from hatching to 28 days of age. -

消 化 管(Digestive Duct)
digestivedigestive systemsystem 刘佳梅刘佳梅 Introduction of digestive system * a long tube extending from the mouth to the anus, and associated with glands. * its main function: -digestion: physical/chemical -absorption * three major sections -the oral cavity including oropharynx -the tubular digestive tract -the major digestive glands: salivary glands, pancreas, liver, general structure of tubular digestive tract basically four layers 1.Mucosa 2.Submucosa 3.Muscularis 4.adventitia Mucosa (tunica mucosa) 1. Epithelium -----two types stratified squamous & simple columnar epith. 2. Lamina propria a layer of C.T. gland capillaries 3. Muscularis mucosa Submucosa *C.T. with small blood / lymphatic vessels; *glands only in the esophagus and duodenum *submucosal plexus *accumulation of lymphatic tissue quite frequently plicae *folds of mucosa and submucosa *longitudinal/circular form. Muscularis *two layers of smooth m. inner layer: circular m. outer layer: longitudinal m. *upper esophagus & the anus with striated m. *three layers in the stomach *between two layers of m. are a vascular plexus and a never plexus Adventitia the outmost layer formed by CT with two form: * fibrosa: CT blending with surrounding structure * serosa: C.T. + mesothelium Esophagus mucosa: stratified squamous epithelium muscularis mucosa: longitudinal bunches of smooth muscle cells submucosa: esophageal glands muscularis: proximal end: skeletal muscle cells distal end: smooth muscle mid portion: mixture muscle Advantitia: fibrosa Esophagogastric junction (longitudinal section) Structural characteristic of stomach *plicae, gastric pits : mucosamucosa *simple columnar epith.: Surface mucous cells * lamina propria :gastric glands muscularis inner layer : oblique m. middle layer : circular m. outer layer : longitudinal m. serosa Three types of gastric glands cardiac glands pyloric glands fundic glands Longitudinal section of fundic gland Cell types: *surface mucous c.