Regulation of Intestinal Blood Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcytosis of Listeria Monocytogenes Across The
Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, Marc Lecuit To cite this version: Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, et al.. Tran- scytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. Journal of Experimental Medicine, Rockefeller University Press, 2011, 208 (11), pp.2263-2277. 10.1084/jem.20110560. pasteur-02040395 HAL Id: pasteur-02040395 https://hal-pasteur.archives-ouvertes.fr/pasteur-02040395 Submitted on 20 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Published Online: 3 October, 2011 | Supp Info: http://doi.org/10.1084/jem.20110560 Downloaded from jem.rupress.org on February 19, 2019 Article Transcytosis of Listeria monocytogenes across -
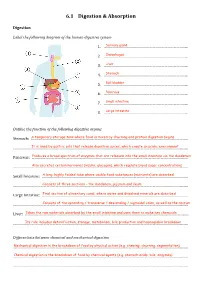
Topic 6.1 Answers
6.1 Digestion & Absorption Digestion Label the following diagram of the human digestive system 1. ………………………………………………………………………...Salivary gland 2. ………………………………………………………………………...Oesophagus 3. ………………………………………………………………………...Liver 4. ………………………………………………………………………...Stomach 5. ………………………………………………………………………...Gall bladder 6. ……Pancreas…………………………………………………………………... 7. ………………………………………………………………………...Small intestine 8. ………………………………………………………………………...Large intestine Outline the function of the following digestive organs Stomach: ………………………………………………………………………………………A temporary storage tank where food is mixed by churning and………………………………………………… protein digestion begins …………………………………………………………………………………………………………………………………………………..........It is lined by gastric pits that release digestive juices, which create an acidic environment Pancreas: …………..……………………………………………………………………………………………………………………………Produces a broad spectrum of enzymes that are released into the small intestine via the duodenum …………………………………………………………………………………………………………………………………………………..........Also secretes certain hormones (insulin, glucagon), which regulate blood sugar concentrations Small Intestine: …………….……………………………………………………………………………………………A long, highly folded tube where usable food substances (nutrients) are absorbed…………………… …………………………………………………………………………………………………………………………………………………..........Consists of three sections – the duodenum, jejunum and ileum Large Intestine: …………….…………………………………………………………………………………………………………………Final section of alimentary canal, where water and dissolved minerals are absorbed …………………………………………………………………………………………………………………………………………………..........Consists -

Modeling Vascular Homeostasis and Improving Data Filtering Methods for Model Calibration
Modeling Vascular Homeostasis and Improving Data Filtering Methods for Model Calibration by Jiacheng Wu A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Engineering − Mechanical Engineering and the Designated Emphasis in Computational Science and Engineering in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Shawn C. Shadden, Chair Professor Grace D. O'Connell Professor Peter L. Bartlett Spring 2019 Modeling Vascular Homeostasis and Improving Data Filtering Methods for Model Calibration Copyright 2019 by Jiacheng Wu 1 Abstract Modeling Vascular Homeostasis and Improving Data Filtering Methods for Model Calibration by Jiacheng Wu Doctor of Philosophy in Engineering − Mechanical Engineering and the Designated Emphasis in Computational Science and Engineering University of California, Berkeley Professor Shawn C. Shadden, Chair Vascular homeostasis is the preferred state that blood vessels try to maintain against external mechanical and chemical stimuli. The vascular adaptive behavior around the homeostatic state is closely related to cardiovascular disease progressions such as arterial aneurysms. In this work, we develop a multi-physics computational framework that couples vascular growth & remodeling (G&R), wall mechanics and hemodynamics to describe the overall vascular adaptive behavior. The coupled simulation is implemented in patient-specific geometries to predict aneurysm progression. Lyapunov stability analysis of the governing -

Tracheal Suctioning Improves Gas Exchange but Not Hemodynamics in Asphyxiated Lambs with Meconium Aspiration
nature publishing group Translational Investigation Articles Tracheal suctioning improves gas exchange but not hemodynamics in asphyxiated lambs with meconium aspiration Satyan Lakshminrusimha1, Bobby Mathew1, Jayasree Nair1, Sylvia F. Gugino1,2, Carmon Koenigsknecht1, Munmun Rawat1, Lori Nielsen1 and Daniel D. Swartz1,2 BACKGROUND: Current neonatal resuscitation guidelines suctioning for vigorous infants (9). Approximately 20–30% recommend tracheal suctioning of nonvigorous neonates of infants born through MSAF are depressed at birth with an born through meconium-stained amniotic fluid. Apgar score of 6 or less at 1 min of age (10). Babies exposed to METHODS: We evaluated the effect of tracheal suctioning at MSAF who have respiratory depression at birth have a higher birth in 29 lambs with asphyxia induced by cord occlusion and incidence of MAS (11). If a baby is born through MSAF, has meconium aspiration during gasping. depressed respirations, decreased muscle tone, and/or a heart RESULTS: Tracheal suctioning at birth (n = 15) decreased rate below 100/min, intubation and direct suctioning of the amount of meconium in distal airways (53 ± 29 particles/mm2 trachea soon after delivery is indicated before breaths have lung area) compared to no suction (499 ± 109 particles/mm2; occurred. There are no randomized, clinical, or translational n = 14; P < 0.001). Three lambs in the suction group had car- studies evaluating the effect of tracheal suctioning on hemody- diac arrest during suctioning, requiring chest compressions namics and gas exchange with perinatal meconium aspiration and epinephrine. Onset of ventilation was delayed in the suc- and asphyxia-induced depression. tion group (146 ± 11 vs. 47 ± 3 s in no-suction group; P = 0.005). -

Clinical Practice Guideline: Red Blood Cell Transfusion in Adult Trauma and Critical Care*
Special Article Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care* Lena M. Napolitano, MD; Stanley Kurek, DO; Fred A. Luchette, MD; Howard L. Corwin, MD; Philip S. Barie, MD; Samuel A. Tisherman, MD; Paul C. Hebert, MD, MHSc; Gary L. Anderson, DO; Michael R. Bard, MD; William Bromberg, MD; William C. Chiu, MD; Mark D. Cipolle, MD; PhD; Keith D. Clancy, MD; Lawrence Diebel, MD; William S. Hoff, MD; K. Michael Hughes, DO; Imtiaz Munshi, MD; Donna Nayduch, RN, MSN, ACNP; Rovinder Sandhu, MD; Jay A. Yelon, MD; for the American College of Critical Care Medicine of the Society of Critical Care Medicine and the Eastern Association for the Surgery of Trauma Practice Management Workgroup Objective: To develop a clinical practice guideline for red blood proved by the EAST Board of Directors, the Board of Regents of the cell transfusion in adult trauma and critical care. ACCM and the Council of SCCM. Design: Meetings, teleconferences and electronic-based com- Results: Key recommendations are listed by category, including munication to achieve grading of the published evidence, discus- (A) Indications for RBC transfusion in the general critically ill patient; sion and consensus among the entire committee members. (B) RBC transfusion in sepsis; (C) RBC transfusion in patients at risk Methods: This practice management guideline was developed by for or with acute lung injury and acute respiratory distress syn- a joint taskforce of EAST (Eastern Association for Surgery of Trauma) drome; (D) RBC transfusion in patients with neurologic injury and the American College of Critical Care Medicine (ACCM) of the and diseases; (E) RBC transfusion risks; (F) Alternatives to RBC Society of Critical Care Medicine (SCCM). -

Inhaled Nitric Oxide and Intravenous Magnesium Sulphate for The
Original Article Singapore Med J 2010; 51(2) : 144 Inhaled nitric oxide and intravenous magnesium sulphate for the treatment of persistent pulmonary hypertension of the newborn Boo N Y, Rohana J, Yong S C, Bilkis A Z, Yong-Junina F ABSTRACT ated with a better outcome than those who Introduction: The aim of this study was to were administered MgSO4 following a failed iNO compare the response and survival rates of term therapy. infants with persistent pulmonary hypertension of the newborn (PPHN) on high frequency oscil- Keywords: inhaled nitric oxide, intravenous latory ventilation (HFOV) treated with either magnesium sulphate, PPHN inhaled nitric oxide (iNO) or intravenous magne- Singapore Med J 2010; 51(2): 144-150 sium sulphate (MgSO4). INTRODUCTION Methods: This was a randomised controlled study. Persistent pulmonary hypertension of the newborn The inclusion criteria were infants with respira- (PPHN) is a complex disorder that is associated tory distress, oxygen index equal to or greater with a wide array of cardiopulmonary diseases and is than 25 despite HFOV support, and echocardio- characterised by marked pulmonary hypertension and graphic evidence of PPHN. Infants in the MgSO4 altered vasoreactivity, leading to the right-to-left shunting group (n is 13) were loaded with MgSO4 200 mg/kg of blood across the patent ductus arteriosus and/or Department of infused over half an hour, followed by continuous foramen ovale. This extrapulmonary shunting associated Paediatics, infusion at 50–150 mg/kg/hour to attain a serum Universiti with high pulmonary vascular resistance causes critical Kebangsaan magnesium level of 5.0–7.0 mmol/L. -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

The Number of Villi in Rat's Jejunum and Ileum: Effect of Normal Growth, Partial Enterectomy, and Tube Feeding
J. Anat. (1972). 111, 2, pp. 283-291 283 With 6 figures Printed in Great Britain The number of villi in rat's jejunum and ileum: effect of normal growth, partial enterectomy, and tube feeding J. M. FORRESTER Department of Physiology, Edinburgh University (Accepted 8 January 1972) INTRODUCTION The villi of the rat small intestine are covered by enterocytes which have a life-span, from their time of origin in the crypts until shedding at the villus tip, of only about one and a half days (Leblond & Stevens, 1948; Bertalanffy, 1960). Their shape varies from one part of the small intestine to another, and even adjacent villi may differ strikingly. In view of these features suggesting a rapidly changing scene, this paper describes a procedure for enumerating the villi in rat jejunum and ileum, and ex- amines the stability of the total number during normal growth, after partial enter- ectomy, and after tube feeding. METHODS Locally bred Wistar male rats were used. They were fed on a standard pelleted rat food manufactured in Edinburgh. They always had tap water ad libitum. Enumeration procedure. Rats were killed by inhalation of chloroform in the morn- ing. The position of the suspensory ligament was marked on the small intestine where a band of connective tissue is attached to the intestine at the duodenojejunal junction. Then the small intestine was removed from pylorus to ileocolic valve by gentle traction, and washed through with cold saline (NaCl 0-9 %, w/v). It was weighed, and after removal of the duodenum, was laid in a trough and perfused with Bouin's solution at an outlet pressure of 20 cm of solution for at least 20 minutes (Hromadkova & Skala, 1969). -

An Electron Microscopic Study of the Intestinal Villus I
An Electron Microscopic Study of the Intestinal Villus I. The Fasting Animal By SANFORD L. PALAY,* M.D., and LEONARD J. KARLIN, M.I). (From the Department of Anatomy, Yale University School of Medicine, New Haven, and the Laboratory of Neuroanalomical Sciences, National Institute of Neurological Diseases and Blindness, National Institutes of Health, Bethesda) PLATES 148 TO 159 (Received for publication, January 16, 1959) ABSTRACT The structure of the intestinal villus of the rat was studied in thin sections of tissue fixed in buffered osmium tetroxide and embedded in methacrylate. The simple columnar epithelium investing the villus is surmounted by a striated border consisting of slender projections of the cell surface. These microvilli are arranged in almost crystalline, hexagonal array, and increase the apical surface area of the cell by a factor of 24. The core of each microvillus is filled with fine fibrils which arise from the filamentous substance of the terminal web underlying the striated border. Each microvillus is covered by a tubular extension of the plasma membrane of the epithelial cell. Pinocytotic vesicles originating from the plasma membrane occur at the bases of the intermicrovillous spaces. The nucleus, mitochondria, and the endoplasmic reticuIum of the epithelial cell display no unusual features. Small bits of ergastoplasm occur in the apical cytoplasm. A thin basement membrane separates the epithelium from the lamina propria which consists of vessels, nerves, and numerous lymphocytes, eosinophiles, mast cells, plasma cells, smooth muscle fibers, and macrophages suspended in a delicate stroma of fibroblasts and collagen fibers. Intercellular fat droplets often occur in this stroma, even in animals fasted for 40 hours. -

Cardiovascular Changes in the Lingcod (Ophiodon Elongatus) Following Adrenergic and Cholinergic Drug Infusions
y. exp. Biol. (1981), 91. 293-3OS 293 With 5 figures in Great Britain CARDIOVASCULAR CHANGES IN THE LINGCOD (OPHIODON ELONGATUS) FOLLOWING ADRENERGIC AND CHOLINERGIC DRUG INFUSIONS BY A. P. FARRELL* Department of Zoology, University of British Columbia, Vancouver, Canada (Received 25 June 1980) SUMMARY Adrenergic and cholinergic agonists were infused into the ventral aorta to evoke gill vasoactivity in the lingcod, Ophiodon elongatus. Arterial blood pres- sures were changed, and cardiac output and stroke volume were increased. As a. consequence both the pressure and flow profiles across the gill were altered, and these changes should alter the pattern of lamellar perfusion. The changes in cardiac function were apparently reflexly mediated. INTRODUCTION The control of lamellar perfusion patterns in fish is not fully understood. Neural and humoral mediated vasoactivities are presumeably important in the control since many investigations have indicated that adrenergic and cholinergic vascular receptors are present in the gills (Wood, 1974, 1975, 1977; Smith, 1977; Payan & Girard, 1977; Dunel & Laurent, 1977). Nevertheless, unequivocal evidence of lamellar innervation or vasoactivity is lacking. Thus neural or humoral control of lamellar arterioles has still to be unequivocally demonstrated. In view of this, it is possible that changes in the pressure and flow profiles across the gill cause important, passive changes in lamellar perfusion. The lamellar blood space and the number of lamellae perfused are pressure and flow dependent in Ophiodon elongatus (Farrell, Daxboeck & Randall, 1979; Farrell et al., 1980) and in Ictalurus punctatus (Holbert, Boland & Olson, 1979). Also, Opdyke, Holcombe & Wilde (1979) concluded for Squalus acanthias that the reduced gill resis- tance associated with an increase in flow was due to passive changes in the branchial vasculature. -

Inomax, INN-Nitric Oxide
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of INOmax. For information on changes after approval please refer to module 8. 1. Introduction Persistent pulmonary hypertension of the new-born (PPHN) is a disorder of the transition from foetal to extra-uterine life, a clinical syndrome characterised by persistence of elevated pulmonary vascular resistance producing right-to-left shunting of deoxygenated blood across the still patent foramen ovale and/or ductus arteriosus as well as systemic hypoxemia. PPHN may be idiopathic or associated with parenchyma lung disease as respiratory distress syndrome of prematurity, meconium aspiration syndrome, pneumonia and sepsis or congenital diaphragmatic hernia and pulmonary hypoplasia. Although anatomical changes such as pulmonary vascular smooth muscle hypertrophy may occur and contribute to increased pulmonary resistance, pulmonary vasoconstriction and altered vascular reactivity are central to the pathophysiology of this syndrome. PPHN is an uncommon life threatening condition (the incidence is less than 0.1% of life births and mortality up to 40%). Conventional therapy consists in hyperoxygenation through mechanical ventilation with hyperoxic gas mixtures, induced metabolic or respiratory alkalosis, deep sedation and pharmacological paralysis to reduce pulmonary vasoactivity. Intravenous vasodilators as sodium nitroprusside or tolazoline may be useful but can lead to severe systemic hypotension, which in turn aggravate the right-to-left shunt. In the most severe forms of hypoxic respiratory failure, invasive procedures of extracorporeal membrane oxygenation (ECMO) have proven efficacy in reducing mortality compared to conventional therapies. However, these techniques remain procedures of exception performed by a trained and specialised neonatology team. They require catheterisation and often ligation of the cervical great vessels (jugular vein and carotid artery) need continuous blood anticoagulation and use of blood products. -
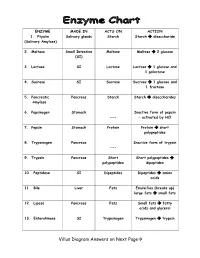
Villus Diagram Answers on Next Page→
ENZYME MADE IN ACTS ON ACTION 1. Ptyalin Salivary glands Starch Starch disaccharide (Salivary Amylase) 2. Maltase Small Intestine Maltose Maltose 2 glucose (SI) 3. Lactase SI Lactose Lactose 1 glucose and 1 galactose 4. Sucrase SI Sucrose Sucrose 1 glucose and 1 fructose 5. Pancreatic Pancreas Starch Starch disaccharides Amylase 6. Pepsinogen Stomach Inactive form of pepsin --- – activated by HCl 7. Pepsin Stomach Protein Protein short polypeptides 8. Trypsinogen Pancreas Inactive form of trypsin --- 9. Trypsin Pancreas Short Short polypeptides polypeptides dipeptides 10. Peptidase SI Dipeptides Dipeptides amino acids 11. Bile Liver Fats Emulsifies (breaks up) large fats small fats 12. Lipase Pancreas Fats Small fats fatty acids and glycerol 13. Enterokinase SI Trypsinogen Trypsinogen trypsin Villus Diagram Answers on Next Page Intestinal villus READ INFORMATION BELOW!!! From Wikipedia, the free encyclopedia Intestinal villi (singular: villus) are tiny, finger-like projections that protrude from the epithelial lining of the intestinal wall. Each villus is approximately 0.5-1.6 mm (millimetres) in length and has many microvilli (singular: microvillus), each of which are much smaller than a single villus. Intestinal villi should not be confused with the larger folds of mucous membrane in the bowel known as the plicae circulares. A villus is much smaller than a single fold of plicae circulares. Villi increase the internal surface area of the intestinal wall. Increased surface area allows for increased intestinal wall area that is available for absorption. Increased absorptive area is useful because digested nutrients (including sugars and amino acids) pass into the villi which is semi permeable, through diffusion, which is effective only at short distances.